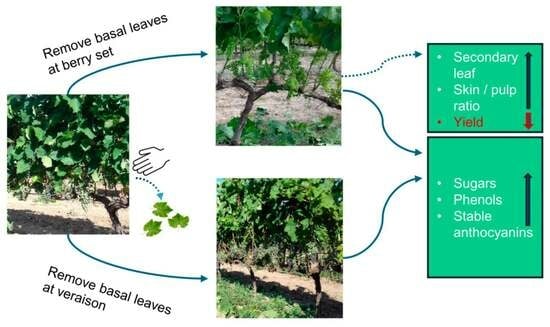
Optimizing ‘Xinomavro’ (Vitis vinifera L.) Performance by Post-Bloom Basal Leaf Removal Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vineyard and Experimental Design
2.2. Stem Water Potential and Leaf Gas Exchange
2.3. Leaf Area and Cluster Temperature Measurement
2.4. Berry Sampling, Must Analysis, and Yield Components
2.5. Total Phenol Content and Anthocyanins
2.6. Statistical Analysis
3. Results & Discussion
3.1. Climatic Conditions and Vine Phenology
3.2. Vine Water Status and Gas Exchange
3.3. Vine Leaf Area and Berry Temperature
3.4. Yield Components
3.5. Total Soluble Solids and Titratable Acidity
3.6. Phenols and Anthocyanins
3.7. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under Deficit Irrigation: Hints from Physiological and Molecular Data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Liu, L.; Cynkar, W.U.; Dambergs, R.G.; Janik, L.; Colby, C.B.; Gishen, M. Effect of Temperature Variation on the Visible and near Infrared Spectra of Wine and the Consequences on the Partial Least Square Calibrations Developed to Measure Chemical Composition. Anal. Chim. Acta 2007, 588, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.-T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine Quality: A Multiple Choice Issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez De Toda, F. Current Viticultural Techniques to Mitigate the Effects of Global Warming on Grape and Wine Quality: A Comprehensive Review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Wang, X.; Lesefko, S.; De Bei, R.; Fuentes, S. Effects of Canopy Management Practices on Grapevine Bud Fruitfulness. OENO One 2020, 54, 313–325. [Google Scholar] [CrossRef]
- Petrie, P.R.; Trought, M.C.T.; Howell, G.S.; Buchan, G.D. The Effect of Leaf Removal and Canopy Height on Whole-Vine Gas Exchange and Fruit Development of Vitis vinifera L. Sauvignon Blanc. Funct. Plant Biol. 2003, 30, 711. [Google Scholar] [CrossRef] [PubMed]
- Intrieri, C.; Filippetti, I.; Allegro, G.; Centinari, M.; Poni, S. Early Defoliation (Hand vs Mechanical) for Improved Crop Control and Grape Composition in Sangiovese (Vitis vinifera L.). Aust. J. Grape Wine Res. 2008, 14, 25–32. [Google Scholar] [CrossRef]
- Bubola, M.; Sivilotti, P.; Janjanin, D.; Poni, S. Early Leaf Removal Has a Larger Effect than Cluster Thinning on Grape Phenolic Composition in Cv. Teran. Am. J. Enol. Vitic. 2017, 68, 234–242. [Google Scholar] [CrossRef]
- Diago, M.P.; Ayestarán, B.; Guadalupe, Z.; Poni, S.; Tardáguila, J. Impact of Prebloom and Fruit Set Basal Leaf Removal on the Flavonol and Anthocyanin Composition of Tempranillo Grapes. Am. J. Enol. Vitic. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Poni, S.; Casalini, L.; Bernizzoni, F.; Civardi, S.; Intrieri, C. Effects of Early Defoliation on Shoot Photosynthesis, Yield Components, and Grape Composition. Am. J. Enol. Vitic. 2006, 57, 397–407. [Google Scholar] [CrossRef]
- Stefanovic, D.; Nikolic, N.; Kostic, L.; Todic, S.; Nikolic, M. Early Leaf Removal Increases Berry and Wine Phenolics in Cabernet Sauvignon Grown in Eastern Serbia. Agronomy 2021, 11, 238. [Google Scholar] [CrossRef]
- Moreno, D.; Vilanova, M.; Gamero, E.; Intrigliolo, D.S.; Talaverano, M.I.; Uriarte, D.; Valdés, M.E. Effects of Preflowering Leaf Removal on Phenolic Composition of Tempranillo in the Semiarid Terroir of Western Spain. Am. J. Enol. Vitic. 2015, 66, 204–211. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight Exposure and Temperature Effects on Berry Growth and Composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar] [CrossRef]
- Gambetta, J.; Romat, V.; Holzapfel, B.; Schmidtke, L. Assessment of Sunburn Damage in Chardonnay Grapes in Relation to Leaf Removal Timing. In Proceedings of the 17th Australian Wine Industry Technical Conference (AWITC 2019), Adelaide, Australia, 21–24 July 2019. [Google Scholar]
- Soar, C.J.; Speirs, J.; Maffei, S.M.; Penrose, A.B.; Mccarthy, M.G.; Loveys, B.R. Grape Vine Varieties Shiraz and Grenache Differ in Their Stomatal Response to VPD: Apparent Links with ABA Physiology and Gene Expression in Leaf Tissue. Aust. J. Grape Wine Res. 2006, 12, 2–12. [Google Scholar] [CrossRef]
- Tardáguila, J.; Diago, M.P.; Martínez De Toda, F.; Poni, S.; Vilanova, M. Effects of Timing of Leaf Removal on Yield, Berry Maturity, Wine Composition and Sensory Properties of Cv. Grenache Grown under Non Irrigated Conditions. OENO One 2008, 42, 221. [Google Scholar] [CrossRef]
- Sabbatini, P.; Howell, G.S. Effects of Early Defoliation on Yield, Fruit Composition, and Harvest Season Cluster Rot Complex of Grapevines. HortScience 2010, 45, 1804–1808. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Georgiadou, A.; Tikos, P.; Kallithraka, S.; Koundouras, S. Effects of Severity of Post-Flowering Leaf Removal on Berry Growth and Composition of Three Red Vitis vinifera L. Cultivars Grown under Semiarid Conditions. J. Agric. Food Chem. 2012, 60, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.C.; Wolf, T.K. Cabernet Sauvignon Responses to Prebloom and Post-Fruit Set Leaf Removal in Virginia. Catal. Discov. Pract. 2018, 2, 24–34. [Google Scholar] [CrossRef]
- VanderWeide, J.; Medina-Meza, I.G.; Frioni, T.; Sivilotti, P.; Falchi, R.; Sabbatini, P. Enhancement of Fruit Technological Maturity and Alteration of the Flavonoid Metabolomic Profile in Merlot (Vitis vinifera L.) by Early Mechanical Leaf Removal. J. Agric. Food Chem. 2018, 66, 9839–9849. [Google Scholar] [CrossRef] [PubMed]
- Wolkovich, E.M.; García De Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the World’s Future Wine-Growing Regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Gkanidi, E.; Koundouras, S.; Mannion, D.T.; Kilcawley, K.N. Discrimination of Five Greek Red Grape Varieties According to the Anthocyanin and Proanthocyanidin Profiles of Their Skins and Seeds. J. Food Compos. Anal. 2020, 92, 103547. [Google Scholar] [CrossRef]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal Phenolic Compounds in Greek Red Wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Taskos, D.G.; Koundouras, S.; Stamatiadis, S.; Zioziou, E.; Nikolaou, N.; Karakioulakis, K.; Theodorou, N. Using Active Canopy Sensors and Chlorophyll Meters to Estimate Grapevine Nitrogen Status and Productivity. Precis. Agric. 2015, 16, 77–98. [Google Scholar] [CrossRef]
- Alatzas, A.; Theocharis, S.; Miliordos, D.-E.; Kotseridis, Y.; Koundouras, S.; Hatzopoulos, P. Leaf Removal and Deficit Irrigation Have Diverse Outcomes on Composition and Gene Expression during Berry Development of Vitis vinifera L. Cultivar Xinomavro. OENO One 2023, 57, 289–305. [Google Scholar] [CrossRef]
- Choné, X. Stem Water Potential Is a Sensitive Indicator of Grapevine Water Status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Lopes, C.; Pinto, P.A. Easy and Accurate Estimation of Grapevine Leaf Area with Simple Mathematical Models. VITIS-J. Grapevine Res. 2015, 44, 55–61. [Google Scholar] [CrossRef]
- Iland, P. Techniques for Chemical Analysis and Quality Monitoring during Winemaking; Patrick Iland Wine Promotions: Campbelltown, Australia, 2000; ISBN 978-0-646-38435-1. [Google Scholar]
- Kyraleou, M.; Kallithraka, S.; Koundouras, S.; Chira, K.; Haroutounian, S.; Spinthiropoulou, H.; Kotseridis, Y. Effect of Vine Training System on the Phenolic Composition of Red Grapes (Vitis vinifera L. Cv. Xinomavro). OENO One 2015, 49, 71. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Trégoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.-P. Vine Water Status Is a Key Factor in Grape Ripening and Vintage Quality for Red Bordeaux Wine. How Can It Be Assessed for Vineyard Management Purposes? OENO One 2009, 43, 121. [Google Scholar] [CrossRef]
- Hunter, J.J.; Hunter, J.J.; Visser, J. The Effect of Partial Defoliation, Leaf Position and Developmental Stage of the Vine on Photosynthetic Activity of Vitis vinifera L. Cv Cabernet Sauvignon. South Afr. J. Enol. Vitic. 1988, 9, 9–15. [Google Scholar] [CrossRef]
- Brandt, M.; Scheidweiler, M.; Rauhut, D.; Patz, C.-D.; Will, F.; Zorn, H.; Stoll, M. The Influence of Temperature and Solar Radiation on Phenols in Berry Skin and Maturity Parameters of Vitis vinifera L. Cv. Riesling. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Caccavello, G.; Giaccone, M.; Scognamiglio, P.; Forlani, M.; Basile, B. Influence of Intensity of Post-Veraison Defoliation or Shoot Trimming on Vine Physiology, Yield Components, Berry and Wine Composition in Aglianico Grapevines: Aglianico Response to Post-Veraison Summer Pruning. Aust. J. Grape Wine Res. 2017, 23, 226–239. [Google Scholar] [CrossRef]
- Petrie, P.; Trought, M.; Howell, G. Influence of Leaf Ageing, Leaf Area and Crop Load on Photosynthesis, Stomatal Conductance and Senescence of Grapevine (Vitis vinifera L. Cv. Pinot Noir) Leaves. Vitis-Geilweilerhof- 2000, 39, 31–36. [Google Scholar]
- Poni, S.; Gatti, M.; Bernizzoni, F.; Civardi, S.; Bobeica, N.; Magnanini, E.; Palliotti, A. Late Leaf Removal Aimed at Delaying Ripening in Cv. Sangiovese: Physiological Assessment and Vine Performance: Late Defoliation and Ripening. Aust. J. Grape Wine Res. 2013, 19, 378–387. [Google Scholar] [CrossRef]
- Bogicevic, M.; Maras, V.; Mugoša, M.; Kodžulović, V.; Raičević, J.; Šućur, S.; Failla, O. The Effects of Early Leaf Removal and Cluster Thinning Treatments on Berry Growth and Grape Composition in Cultivars Vranac and Cabernet Sauvignon. Chem. Biol. Technol. Agric. 2015, 2, 13. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of Sunlight and Temperature Effects on the Composition of Vitis vinifera Cv. Merlot Berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar] [CrossRef]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Belcher, S.; Lorenzini, F.; Rösti, J.; Koestel, C.; Gindro, K.; Spring, J.-L. Intensity and Timing of Defoliation on White Cultivar Chasselas under the Temperate Climate of Switzerland. OENO One 2018, 52, 93–104. [Google Scholar] [CrossRef]
- Ivanišević, D.; Kalajdžić, M.; Drenjančević, M.; Puškaš, V.; Korać, N. The Impact of Cluster Thinning and Leaf Removal Timing on the Grape Quality and Concentration of Monomeric Anthocyanins in Cabernet-Sauvignon and Probus (Vitis vinifera L.) Wines. OENO One 2020, 54, 63–74. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Buesa, I.; Yeves, A.; Pérez, D.; Risco, D.; Castel, J.R.; Intrigliolo, D.S. Unravelling the Effects of Berry Size on ‘Tempranillo’ Grapes under Different Field Practices. Ciênc. E Téc. Vitivinícola 2019, 34, 1–14. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147. [Google Scholar] [CrossRef]
- Koundouras, S.; Hatzidimitriou, E.; Karamolegkou, M.; Dimopoulou, E.; Kallithraka, S.; Tsialtas, J.T.; Zioziou, E.; Nikolaou, N.; Kotseridis, Y. Irrigation and Rootstock Effects on the Phenolic Concentration and Aroma Potential of Vitis vinifera L. Cv. Cabernet Sauvignon Grapes. J. Agric. Food Chem. 2009, 57, 7805–7813. [Google Scholar] [CrossRef] [PubMed]
- Melino, V.J.; Hayes, M.A.; Soole, K.L.; Ford, C.M. The Role of Light in the Regulation of Ascorbate Metabolism during Berry Development in the Cultivated Grapevine Vitis vinifera L.: Regulation of Ascorbate Metabolism in Developing Berries. J. Sci. Food Agric. 2011, 91, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, F.; Reynolds, A.G.; Kasimos, A. Canopy Management and Enzyme Impacts on Merlot, Cabernet Franc, and Cabernet Sauvignon. I. Yield and Berry Composition. Am. J. Enol. Vitic. 2011, 62, 139–151. [Google Scholar] [CrossRef]
- Matus, J.T.; Loyola, R.; Vega, A.; Peña-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, J.A. Post-Veraison Sunlight Exposure Induces MYB-Mediated Transcriptional Regulation of Anthocyanin and Flavonol Synthesis in Berry Skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of Different Cluster Sunlight Exposure Levels on Ripening and Anthocyanin Accumulation in Nebbiolo Grapes. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of Anthocyanins in Red-Wine Grape under High Temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Downey, M.O.; Iland, P.G.; Bindon, K.; Francis, I.L.; Herderich, M.; Robinson, S.P. Exclusion of Sunlight from Shiraz Grapes Alters Wine Colour, Tannin and Sensory Properties. Aust. J. Grape Wine Res. 2007, 13, 53–65. [Google Scholar] [CrossRef]
- Rinaldo, A.; Cavallini, E.; Jia, Y.; Moss, S.M.A.; McDavid, D.A.J.; Hooper, L.C.; Robinson, S.P.; Tornielli, G.B.; Zenoni, S.; Ford, C.M.; et al. A Grapevine Anthocyanin Acyltransferase, Transcriptionally Regulated by VvMYBA, Can Produce Most Acylated Anthocyanins Present in Grape Skins. Plant Physiol. 2015, 169, 1897–1916. [Google Scholar] [CrossRef] [PubMed]








| Year | Month | Mean Temp. | Max Temp. | Min Temp. | Rainfall |
|---|---|---|---|---|---|
| 2015 | April | 13.5 | 20.0 | 7.1 | 14.4 |
| May | 20.1 | 26.9 | 13.5 | 19.8 | |
| June | 23.2 | 29.8 | 17.1 | 96.2 | |
| July | 27.5 | 34.3 | 20.5 | 8.2 | |
| August | 27.1 | 33.8 | 20.4 | 33.4 | |
| September | 23.7 | 29.7 | 18.8 | 129.6 | |
| 2016 | April | 17.1 | 22.5 | 12.3 | 7.8 |
| May | 19.5 | 25.9 | 12.8 | 73.0 | |
| June | 26.0 | 32.5 | 19.0 | 15.2 | |
| July | 27.9 | 34.4 | 21.1 | 1.2 | |
| August | 27.0 | 33.5 | 20.8 | 25.8 | |
| September | 21.7 | 27.9 | 16.2 | 88.8 | |
| 2017 | April | 14.5 | 21.5 | 7.7 | 8.8 |
| May | 19.8 | 26.7 | 13.1 | 48.6 | |
| June | 25.9 | 32.9 | 18.8 | 8.8 | |
| July | 27.1 | 32.9 | 21.6 | 52.2 | |
| August | 27.1 | 31.7 | 22.7 | 34.8 | |
| September | 22.9 | 27.7 | 18.9 | 13.4 | |
| Average April to September | 2015 | 22.5 | 29.1 | 16.3 | 301.6 |
| 2016 | 23.2 | 29.5 | 17.1 | 211.8 | |
| 2017 | 22.9 | 28.9 | 17.2 | 182.6 |
| Year | Treatment | Main Leaf Area (m2/vine) | Sec. Leaf Area (m2/vine) | Berry Temp. (°C) | Differ. Air-Berry Temp. (°C) |
|---|---|---|---|---|---|
| 2015 | BS | 1.5 c | 1.76 a | 28.8 b | 3.4 b |
| VR | 2.2 b | 1.08 b | 29.2 a | 3.2 b | |
| CO | 2.7 a | 0.42 c | 27.9 c | 4.7 a | |
| 2016 | BS | 1.3 c | 1.77 a | 29.0 a | 3.2 b |
| VR | 2.1 b | 0.78 b | 29.3 a | 3.3 b | |
| CO | 2.8 a | 0.32 c | 27.8 b | 4.9 a | |
| 2017 | BS | 1.4 c | 1.83 a | 29.0 a | 2.7 b |
| VR | 2.1 b | 0.99 b | 28.3 ab | 3.2 b | |
| CO | 2.6 a | 0.55 c | 27.6 b | 4.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theocharis, S.; Taskos, D.; Gkrimpizis, T.; Nikolaou, K.-E.; Miliordos, D.-E.; Koundouras, S. Optimizing ‘Xinomavro’ (Vitis vinifera L.) Performance by Post-Bloom Basal Leaf Removal Applications. Horticulturae 2024, 10, 340. https://doi.org/10.3390/horticulturae10040340
Theocharis S, Taskos D, Gkrimpizis T, Nikolaou K-E, Miliordos D-E, Koundouras S. Optimizing ‘Xinomavro’ (Vitis vinifera L.) Performance by Post-Bloom Basal Leaf Removal Applications. Horticulturae. 2024; 10(4):340. https://doi.org/10.3390/horticulturae10040340
Chicago/Turabian StyleTheocharis, Serafeim, Dimitrios Taskos, Theodoros Gkrimpizis, Kleopatra-Eleni Nikolaou, Dimitrios-Evangelos Miliordos, and Stefanos Koundouras. 2024. "Optimizing ‘Xinomavro’ (Vitis vinifera L.) Performance by Post-Bloom Basal Leaf Removal Applications" Horticulturae 10, no. 4: 340. https://doi.org/10.3390/horticulturae10040340