Coordinated Expression of the Genes Encoding FocA and Pyruvate Formate-Lyase Is Important for Maintenance of Formate Homeostasis during Fermentative Growth of Escherichia coli
Abstract
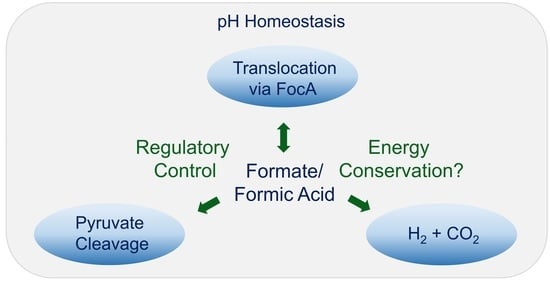
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Determination of β-Galactosidase Enzyme Activity
2.3. Measurement of Extracellular Formate Concentration
3. Results
3.1. FocA Offers a Growth Advantage during Early Exponential Phase Growth
3.2. Increasing the Copy Number of focA Does Not Affect Intracellular Formate Levels in the Parental Strain
3.3. Increased Synthesis of Chromosomally Encoded Native FocA Does Not Significantly Affect the Intracellular Formate Concentration
3.4. Co-Expression of focA and pflB Is Important in Determining Formate Homeostatic Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Böck, A.; Sawers, G. Fermentation. In Escherichia coli and Salmonella: Molecular and Cellular Biology, 2nd ed.; Neidhardt, F.C., Curtiss, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.F., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; American Society for Microbiology Press: Washington, DC, USA, 1996; Volume 1, pp. 262–282. [Google Scholar]
- Noguchi, K.; Riggins, D.P.; Eldahan, K.C.; Kitko, R.D.; Slonczewski, J.L. Hydrogenase-3 Contributes to Anaerobic Acid Resistance of Escherichia coli. PLoS ONE 2010, 5, e10132. [Google Scholar] [CrossRef]
- Kammel, M.; Pinske, C.; Sawers, R.G. FocA and its central role in fine-tuning pH homeostasis of enterobacterial formate metabolism. Microbiology 2022, 168, 001253. [Google Scholar] [CrossRef]
- Sargent, F. The model [NiFe]-hydrogenases of Escherichia coli. Adv. Microb. Physiol. 2016, 68, 433–507. [Google Scholar]
- Pinske, C.; Sawers, R.G. Anaerobic formate and hydrogen metabolism. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Peters, K.; Sargent, F. Formate hydrogenlyase, formic acid translocation and hydrogen production: Dynamic membrane biology during fermentation. Biochim. Biophys. Acta Bioenerg. 2023, 1864, 148919. [Google Scholar] [CrossRef]
- Steinhilper, R.; Höff, G.; Heider, J.; Murphy, B.J. Structure of the membrane-bound formate hydrogenlyase complex from Escherichia coli. Nat. Commun. 2022, 13, 5395. [Google Scholar] [CrossRef]
- Knappe, J.; Sawers, G. A radical-chemical route to acetyl-CoA: The anaerobically induced pyruvate formate-lyase system of Escherichia coli. FEMS Microbiol. Rev. 1990, 6, 383–398. [Google Scholar]
- Suppmann, B.; Sawers, G. Isolation and characterisation of hypophosphite-resistant mutants of Escherichia coli: Identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol. Microbiol. 1994, 11, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Beyer, L.; Doberenz, C.; Falke, D.; Hunger, D.; Suppmann, B.; Sawers, R.G. Coordinating FocA and pyruvate formate-lyase synthesis in Escherichia coli: Preferential translocation of formate over other mixed-acid fermentation products. J. Bacteriol. 2013, 195, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Wang, J.; Cheng, C.; Huang, W.; Lu, P.; Xu, Y.-N.; Wang, P.; Yan, N.; Shi, Y. Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature 2009, 462, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kammel, M.; Hunger, D.; Sawers, R.G. The soluble cytoplasmic N-terminal domain of the FocA channel gates bidirectional formate translocation. Mol. Microbiol. 2021, 115, 758–773. [Google Scholar] [CrossRef]
- Kammel, M.; Trebbin, O.; Pinske, C.; Sawers, R.G. A single amino acid exchange converts FocA into a unidirectional efflux channel for formate. Microbiology 2022, 168, 001132. [Google Scholar] [CrossRef] [PubMed]
- Kammel, M.; Sawers, R.G. The FocA channel functions to maintain intracellular formate homeostasis during Escherichia coli fermentation. Microbiology 2022, 168, 001168. [Google Scholar] [CrossRef] [PubMed]
- Pakes, W.C.C.; Jollyman, W.H. The bacterial decomposition of formic acid into carbon dioxide and hydrogen. J. Chem. Soc. Trans. 1901, 79, 386–391. [Google Scholar] [CrossRef]
- Stephenson, M.; Stickland, L.H. Hydrogenlyases: Bacterial enzymes liberating molecular hydrogen. Biochem. J. 1932, 26, 712–724. [Google Scholar] [CrossRef]
- Waight, A.B.; Love, J.; Wang, D.-N. Structure and mechanism of a pentameric formate channel. Nat. Struct. Mol. Biol. 2010, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lü, W.; Du, J.; Wacker, T.; Gerbig-Smentek, E.; Andrade, S.L.A.; Einsle, O. pH-dependent gating in a FocA formate channel. Science 2011, 332, 352–354. [Google Scholar] [CrossRef]
- Kammel, M.; Trebbin, O.; Sawers, R.G. Interplay between the conserved pore residues Thr-91 and His-209 controls formate translocation through the FocA channel. Microb. Physiol. 2022, 32, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, G.D.; Sargent, F.; Hippler, M. Hydrogen production in the presence of oxygen by Escherichia coli K-12. Microbiology 2022, 168, 001167. [Google Scholar] [CrossRef]
- Vivijs, B.; Haberbeck, L.U.; Mbong, V.B.M.; Bernaerts, K.; Geeraerd, A.H.; Aertsen, A.; Michiels, C.W. Formate hydrogenlyase mediates stationary-phase deacidification and increases survival during sugar fermentation in acetoin-producing enterobacteria. Front. Microbiol. 2015, 6, 150. [Google Scholar] [CrossRef]
- Birkmann, A.; Zinoni, F.; Sawers, G.; Bock, A. Factors affecting transcriptional regulation of the formate hydrogen-lyase pathway of Escherichia coli. Arch. Microbiol. 1987, 148, 44–51. [Google Scholar] [CrossRef]
- Rossmann, R.; Sawers, G.; Böck, A. Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate and pH: Definition of the formate regulon. Mol. Microbiol. 1991, 5, 2807–2814. [Google Scholar] [CrossRef]
- Hopper, S.; Böck, A. Effector-mediated stimulation of ATPase activity by the sigma 54-dependent transcriptional activator FHLA from Escherichia coli. J. Bacteriol. 1995, 177, 2798–2803. [Google Scholar] [CrossRef]
- Leonhardtsberger, S.; Korsa, I.; Böck, A. The molecular biology of formate metabolism in enterobacteria. J. Mol. Microbiol. Biotechnol. 2002, 4, 269–276. [Google Scholar]
- Doberenz, C.; Zorn, M.; Falke, D.; Nannemann, D.; Hunger, D.; Beyer, L.; Ihling, C.H.; Meiler, J.; Sinz, A.; Sawers, R.G. Pyruvate formate-lyase interacts directly with the formate channel FocA to regulate formate translocation. J. Mol. Biol. 2014, 426, 2827–2839. [Google Scholar] [CrossRef]
- Sawers, G.; Böck, A. Novel transcriptional control of the pyruvate formate-lyase gene: Upstream regulatory sequences and multiple promoters regulate anaerobic expression. J. Bacteriol. 1989, 171, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Sawers, R.G. Evidence for novel processing of the anaerobically inducible dicistronic focA-pfl mRNA transcript in Escherichia coli. Mol. Microbiol. 2005, 58, 1441–1453. [Google Scholar] [CrossRef]
- Hunger, D.; Doberenz, C.; Sawers, R.G. Identification of key residues in the formate channel FocA that control import and export of formate. Biol. Chem. 2014, 395, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Casadaban, M.J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 1976, 104, 541–555. [Google Scholar] [CrossRef]
- Christiansen, L.; Pedersen, S. Cloning, restriction endonuclease mapping and posttranscriptional regulation of rspA, the structural gene for ribosomal protein S1. Mol. Gen. Genet. 1981, 181, 548–551. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Miller, J. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Falke, D.; Schulz, K.; Doberenz, C.; Beyer, L.; Lilie, H.; Thiemer, B.; Sawers, R.G. Unexpected oligomeric structure of the FocA formate channel of Escherichia coli: A paradigm for the formate-nitrite transporter family of integral membrane proteins. FEMS Microbiol. Lett. 2010, 303, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pecher, A.; Blaschkowski, H.P.; Knappe, J.; Böck, A. Expression of pyruvate formate-lyase of Escherichia coli from the cloned structural gene. Arch. Microbiol. 1982, 132, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Merlin, C.; Masters, M.; McAteer, S.; Coulson, A. Why is carbonic anhydrase essential to Escherichia coli? J. Bacteriol. 2003, 185, 6415–6424. [Google Scholar] [CrossRef] [PubMed]




| Strains and Plasmids | Relevant Genotype or Characteristics | Reference or Source |
|---|---|---|
| Strains | ||
| MC4100 | F− araD Δ(argF lac) U 169 ptsF25 deoC1 relA1 fblB530 rpsL 150 λ− | [30] |
| DH4100 | MC4100 λ(fdhF::lacZ)—parental strain | [29] |
| DH701 | REK701 λ(fdhF::lacZ)—has two stop codons at codon positions 114 and 115 in the focA gene; the focA gene is present but the strain cannot make FocA | [29] |
| DH702 | REK703 λ(fdhF::lacZ)—chromosomal GTG→ATG exchange in focA; overproduces FocA | [13] |
| DH4200 | MC4200 λ(fdhF::lacZ—has a chromosomal exchange at codon 209 (His→Asn) in the focA gene; FocAH209N has an efflux-only phenotype | [13] |
| DH4300 | MC4300 λ(fdhF::lacZ)—chromosomal exchange at codon 91 (Thr→Ala) in the focA gene; FocAT91A has an efflux-defective phenotype | [14] |
| Plasmids | ||
| pfocA | Ampr, expression vector with the gene focA(without StrepII tag) | [13] |
| pfocA-T91A | Similar to pfocA, but codon threonine 91 exchanged for alanine | [19] |
| pfocA-H209D | Similar to pfocA, but codon histidine 209 exchanged for aspartic acid | [19] |
| pfocA-H209N | Similar to pfocA, but codon histidine 209 exchanged for asparagine | [13] |
| p29 | Cmr, focA+ pflB+ pflA+ | [27,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammel, M.; Sawers, R.G. Coordinated Expression of the Genes Encoding FocA and Pyruvate Formate-Lyase Is Important for Maintenance of Formate Homeostasis during Fermentative Growth of Escherichia coli. Fermentation 2023, 9, 382. https://doi.org/10.3390/fermentation9040382
Kammel M, Sawers RG. Coordinated Expression of the Genes Encoding FocA and Pyruvate Formate-Lyase Is Important for Maintenance of Formate Homeostasis during Fermentative Growth of Escherichia coli. Fermentation. 2023; 9(4):382. https://doi.org/10.3390/fermentation9040382
Chicago/Turabian StyleKammel, Michelle, and Robert Gary Sawers. 2023. "Coordinated Expression of the Genes Encoding FocA and Pyruvate Formate-Lyase Is Important for Maintenance of Formate Homeostasis during Fermentative Growth of Escherichia coli" Fermentation 9, no. 4: 382. https://doi.org/10.3390/fermentation9040382