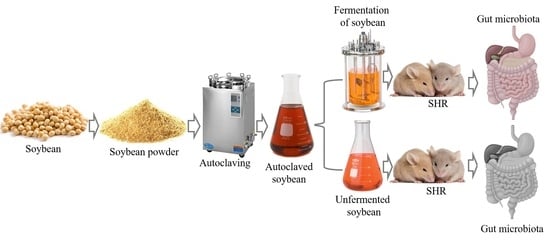
Lacto-Fermented and Unfermented Soybean Differently Modulate Serum Lipids, Blood Pressure and Gut Microbiota during Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Cultures
2.2. Soybean Fermentation
2.3. In Vitro ACE Inhibitory Effects of Fermented Soybean (FSB) and Unfermented Soybean (USB) Samples
- B = Abz in sample based on standard curve slope (pmol).
- ΔT = reaction time (minutes),
- P = sample used into the reaction well (in mg),
- D = sample dilution factor
2.4. Animal Handling and Preparation
2.5. Antihypertensive Effects of FSB and USB
2.6. Effects of FSB and USB Consumption on Serum ACE and Atherogenic Lipids
2.7. Chromatographic Analysis of USB and FSB for Antihypertensive Compounds
2.8. Effects of FSB and USB on the Gut Microbiota (Next Generation Sequencing)
2.9. Data and Statistical Analyses
3. Results
3.1. In Vitro ACE Inhibitory Activity of FSB and USB
3.2. Impact of FSB and USB Consumption on High Blood Pressure and Atherogenic Lipids
3.3. Antihypertensive Compounds in USB and FSB
3.4. Impact of FSB and USB Consumption on Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrario, C.M.; Groban, L.; Wang, H.; Sun, X.; VonCannon, J.L.; Wright, K.N.; Ahmad, S.J.K.I.S. The renin–angiotensin system biomolecular cascade: A 2022 update of newer insights and concepts. Kidney Int. Suppl. 2022, 12, 36–47. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H.; Park, M.H.; Kim, J.-H.; Oh, D.-H.J.L. Novel angiotensin I-converting enzyme inhibitory peptides from soybean protein isolates fermented by Pediococcus pentosaceus SDL1409. LWT–Food Sci. Technol. 2018, 93, 88–93. [Google Scholar] [CrossRef]
- Saidu, H.; Karaye, K.M.; Okeahialam, B.N. Plasma lipid profile in Nigerians with high-normal blood pressure. BMC Res. Notes 2014, 7, 930. [Google Scholar] [CrossRef]
- Jaworska, K.; Huc, T.; Samborowska, E.; Dobrowolski, L.; Bielinska, K.; Gawlak, M.; Ufnal, M.J. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE 2017, 12, e0189310. [Google Scholar] [CrossRef] [PubMed]
- Nagatake, T.; Hirata, S.-I.; Koga, T.; Kuroda, E.; Kobari, S.; Suzuki, H.; Hosomi, K.; Matsumoto, N.; Yanrismet, Y.; Shimojou, M.J.; et al. BLT1 mediates commensal bacteria-dependent innate immune signals to enhance antigen-specific intestinal IgA responses. Mucosal Immunol. 2019, 12, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef]
- Zuo, K.; Li, J.; Xu, Q.; Hu, C.; Gao, Y.; Chen, M.; Hu, R.; Liu, Y.; Chi, H.; Yin, Q.J.C. Dysbiotic gut microbes may contribute to hypertension by limiting vitamin D production. Clin. Cardiol. 2019, 42, 710–719. [Google Scholar] [CrossRef]
- Silveira-Nunes, G.; Durso, D.F.; de Oliveira, L.R.A., Jr.; Cunha, E.H.M.; Maioli, T.U.; Vieira, A.T.; Speziali, E.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.J. Hypertension is associated with intestinal microbiota dysbiosis and inflammation in a Brazilian population. Front. Pharmacol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Nakai, M.; Ribeiro, R.V.; Stevens, B.R.; Gill, P.; Muralitharan, R.R.; Yiallourou, S.; Muir, J.; Carrington, M.; Head, G.A.; Kaye, D.M. Essential hypertension is associated with changes in gut microbial metabolic pathways: A multisite analysis of ambulatory blood pressure. Hypertension 2021, 78, 804–815. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Lee, B.H.; An, H.; Elahi, F.; Barathikannan, K.; Kim, J.-H.; Oh, D.-H. Influence of fermented soy protein consumption on hypertension and gut microbial modulation in spontaneous hypertensive rats. Biosci. Microbiota Food Health 2020, 39, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A.J.N. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M.J. Potential TMA-producing bacteria are ubiquitously found in mammalia. Front. Microbiol. 2020, 10, 2966. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.J.R. Gut microbiota dependent trimethylamine n-oxide aggravates angiotensin II–induced hypertension. Redox. Biol. 2021, 46, 102115. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Song, Q.; Yu, Q.; Chen, Y.; Hong, Y.; Shen, M.J. Dietary polysaccharide from mung bean [Vigna radiate (linn.) wilczek] skin modulates gut microbiota and short-chain fatty acids in mice. Int. J. Food Sci. Technol. 2022, 57, 2581–2589. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T.J.P. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Mosallanezhad, Z.; Mahmoodi, M.; Ranjbar, S.; Hosseini, R.; Clark, C.C.; Carson-Chahhoud, K.; Norouzi, Z.; Abbasian, A.; Sohrabi, Z.; Jalali, M.J.C. Soy intake is associated with lowering blood pressure in adults: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Complement. Ther. Med. 2021, 59, 102692. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Kim, S.H.; Park, B.J.; Kim, H.S.; Kim, J.M.; Kim, H.S.; Oh, D.H. Effects of different processing methods on the antioxidant and immune stimulating abilities of garlic. Food Sci. Nutr. 2019, 7, 1222–1229. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Tyagi, A.; Ofosu, F.K.; Chelliah, R.; Kim, J.-H.; Kim, J.-R.; Yoo, D.; Oh, D.-H. A discovery-based metabolomic approach using UHPLC Q-TOF MS/MS unveils a plethora of prospective antihypertensive compounds in Korean fermented soybeans. LWT–Food Sci. Technol. 2021, 137, 110399. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. Beneficial effects of mung bean seed coat on the prevention of high-fat diet-induced obesity and the modulation of gut microbiota in mice. Eur. J. Nutr. 2021, 60, 2029–2045. [Google Scholar] [CrossRef]
- Kong, C.-Y.; Li, Z.-M.; Mao, Y.-Q.; Chen, H.-L.; Hu, W.; Han, B.; Wang, L.-S. Probiotic yogurt blunts the increase of blood pressure in spontaneously hypertensive rats via remodeling of the gut microbiota. Food Funct. 2021, 12, 9773–9783. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Hori, K.; Shinbo, M.; Hiwatashi, K.; Gotoh, T.; Yamada, S.J.B. Isolation of human renin inhibitor from soybean: Soyasaponin I is the novel human renin inhibitor in soybean. Biosci. Biotechnol. Biochem. 2008, 72, 3232–3236. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Daliri, E.B.-M.; Oh, D.H. Screening for potential probiotic bacteria from Korean fermented soybean paste: In vitro and Caenorhabditis elegans model testing. LWT–Food Sci. Technol. 2018, 88, 132–138. [Google Scholar] [CrossRef]
- Zhang-James, Y.; Middleton, F.A.; Faraone, S.V. Genetic architecture of Wistar-Kyoto rat and spontaneously hypertensive rat substrains from different sources. Physiol. Genom. 2013, 45, 528–538. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Kim, J.-H.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Untargeted metabolomics of fermented rice using UHPLC Q-TOF MS/MS reveals an abundance of potential antihypertensive compounds. Foods 2020, 9, 1007. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Rasmussen, E.G.; Maheshwari, V.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Raman spectroscopic data from formic acid decomposition in subcritical and supercritical water. Data Br. 2020, 29, 105312. [Google Scholar] [CrossRef]
- Godos, J.; Sinatra, D.; Blanco, I.; Mulè, S.; La Verde, M.; Marranzano, M. Association between dietary phenolic acids and hypertension in a Mediterranean cohort. Nutrients 2017, 9, 1069. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, K.; Singh Chauhan, J.; Nagpal, G.; Kumar, R.; Sharma, M.; Raghava, G.P.S. An in silico platform for predicting, screening and designing of antihypertensive peptides. Sci. Rep. 2015, 5, 12512. [Google Scholar] [CrossRef]
- García-Contreras, R.; Vos, P.; Westerhoff, H.V.; Boogerd, F.C. Why in vivo may not equal in vitro–new effectors revealed by measurement of enzymatic activities under the same in vivo-like assay conditions. FEBS J. 2012, 279, 4145–4159. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Park, M.H.; Kim, J.-H.; Oh, D.-H. Development of a soy protein hydrolysate with an antihypertensive effect. Int. J. Mol. Sci. 2019, 20, 1496. [Google Scholar] [CrossRef] [Green Version]
- Shin, Z.-I.; Yu, R.; Park, S.-A.; Chung, D.K.; Ahn, C.-W.; Nam, H.-S.; Kim, K.-S.; Lee, H.J.J. His-His-Leu, an angiotensin I converting enzyme inhibitory peptide derived from Korean soybean paste, exerts antihypertensive activity in vivo. J. Agric. Food Chem. 2001, 49, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Mun, E.-G.; Wang, J.-X.; Cha, Y.-S.J.F. Chinese traditional fermented soy sauce exerts protective effects against high-fat and high-salt diet-induced hypertension in sprague-dawley rats by improving adipogenesis and renin-angiotensin-aldosterone system activity. Fermentation 2021, 7, 52. [Google Scholar] [CrossRef]
- Banjoko, I.O.; Adeyanju, M.M.; Ademuyiwa, O.; Adebawo, O.O.; Olalere, R.A.; Kolawole, M.O.; Adegbola, I.A.; Adesanmi, T.A.; Oladunjoye, T.O.; Ogunnowo, A.A.; et al. Hypolipidemic effects of lactic acid bacteria fermented cereal in rats. Lipids Health Dis. 2012, 11, 170. [Google Scholar] [CrossRef]
- Du, J.; Li, J.-M.J.H. BAS/BSCR23 apocynin treatment reduces high-fat diet-induced obesity and hypertension but has no significant effect on hyperglycaemia. Heart 2010, 96, e19. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Gao, L.; Zheng, X.; Feng, W. Acacetin improves endothelial dysfunction and aortic fibrosis in insulin-resistant shr rats by estrogen receptors. Mol. Biol. Rep. 2020, 47, 6899–6918. [Google Scholar] [CrossRef] [PubMed]
- Chamata, Y.; Watson, K.A.; Jauregi, P. Whey-derived peptides interactions with ACE by molecular docking as a potential predictive tool of natural ACE inhibitors. Int. J. Mol. Sci. 2020, 21, 864. [Google Scholar] [CrossRef]
- Siddegowda, G.S.; Bhaskar, N.; Gopal, S. Fermentative properties of proteolytic Pediococcus strains isolated from salt fermented fish hydrolysate prepared using freshwater fish rohu (Labeo rohita). J. Aquat. Food Prod. Technol. 2017, 26, 341–355. [Google Scholar] [CrossRef]
- Singh, B.M.; Mehta, J.L. Interactions Between the renin-angiotensin system and dyslipidemia: Relevance in the therapy of hypertension and coronary heart disease. Arch. Intern. Med. 2003, 163, 1296–1304. [Google Scholar] [CrossRef]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, gen. Nov., sp. Nov., A saccharolytic butyrate-producer within the family lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef]
- Hadinia, N.; Edalatian Dovom, M.R.; Yavarmanesh, M. The effect of fermentation conditions (temperature, salt concentration, and ph) with Lactobacillus strains for producing short chain fatty acids. LWT–Food Sci. Technol. 2022, 165, 113709. [Google Scholar] [CrossRef]
- Li, Z.; Hu, G.; Zhu, L.; Sun, Z.; Jiang, Y.; Gao, M.J.; Zhan, X. Study of growth, metabolism, and morphology of Akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Iwasaki, Y.; Yada, T.J. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 2018, 57, 130–135. [Google Scholar] [CrossRef]
- Saku, K.; Kishi, T.; Sakamoto, K.; Hosokawa, K.; Sakamoto, T.; Murayama, Y.; Kakino, T.; Ikeda, M.; Ide, T.; Sunagawa, K. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiol. Rep. 2014, 2, e12136. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.P.; Walter, J.; Tannock, G.W.; Tonkonogy, S.L.; Sartor, B.R. Bifidobacterium animalis causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin-10-deficient mice. Inflamm. Bowel Dis. 2009, 15, 1022–1031. [Google Scholar] [CrossRef]
- Zegarra-Ruiz, D.F.; El Beidaq, A.; Iñiguez, A.J.; Di Ricco, M.L.; Vieira, S.M.; Ruff, W.E.; Mubiru, D.; Fine, R.L.; Sterpka, J.; Greiling, T.M.J.C.h.; et al. A diet-sensitive commensal lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 2019, 25, 113–127.e6. [Google Scholar] [CrossRef]
- Priya, S.; Burns, M.B.; Ward, T.; Mars, R.A.T.; Adamowicz, B.; Lock, E.F.; Kashyap, P.C.; Knights, D.; Blekhman, R. Identification of shared and disease-specific host gene–microbiome associations across human diseases using multi-omic integration. Nat. Microbiol. 2022, 7, 780–795. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Oh, D.-H. Lacto-Fermented and Unfermented Soybean Differently Modulate Serum Lipids, Blood Pressure and Gut Microbiota during Hypertension. Fermentation 2023, 9, 152. https://doi.org/10.3390/fermentation9020152
Daliri EB-M, Ofosu FK, Chelliah R, Oh D-H. Lacto-Fermented and Unfermented Soybean Differently Modulate Serum Lipids, Blood Pressure and Gut Microbiota during Hypertension. Fermentation. 2023; 9(2):152. https://doi.org/10.3390/fermentation9020152
Chicago/Turabian StyleDaliri, Eric Banan-Mwine, Fred Kwame Ofosu, Ramachandran Chelliah, and Deog-Hwan Oh. 2023. "Lacto-Fermented and Unfermented Soybean Differently Modulate Serum Lipids, Blood Pressure and Gut Microbiota during Hypertension" Fermentation 9, no. 2: 152. https://doi.org/10.3390/fermentation9020152