Sediment Microbial Fuel Cells with Algae-Assisted Cathodes for Electricity Generation and Bio-Treatment of Sewage Sludge
Abstract
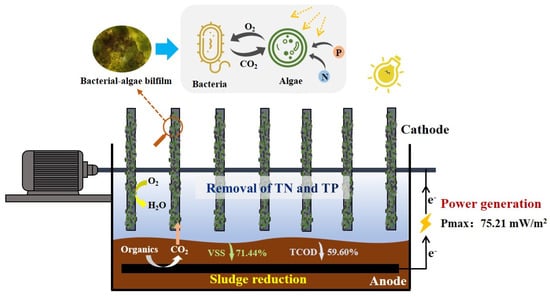
:1. Introduction
2. Materials and Methods
2.1. Construction of SMFCs
2.2. Operating Conditions
2.3. Electrochemical and Chemical Analysis Methods
2.4. Microbial Community Analysis
3. Results and Discussion
3.1. Electrochemical Performance of SMFCs
3.2. Organic Matter Removal Efficiency in the Sediment of the SMFCs
3.3. Variation of Nitrogen and Phosphorus Concentrations in SMFCs
3.4. Characterization of the EPS of the Sediment Sludge
3.4.1. Content and Composition of the EPS
3.4.2. Three-Dimensional Fluorescence Spectra of the EPS
3.5. Bacterial Community Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saby, S.; Djafer, M.; Chen, G.-H. Feasibility of using a chlorination step to reduce excess sludge in activated sludge process. Water Res. 2002, 36, 656–666. [Google Scholar] [CrossRef]
- Wang, G.; Sui, J.; Shen, H.; Liang, S.; He, X.; Zhang, M.; Xie, Y.; Li, L.; Hu, Y. Reduction of excess sludge production in sequencing batch reactor through incorporation of chlorine dioxide oxidation. J. Hazard. Mater. 2011, 192, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, S.; Lu, X.; Xi, B.; Guo, X.; Wang, H.; Duan, J. Excess sludge reduction using pilot-scale lysis-cryptic growth system integrated ultrasonic/alkaline disintegration and hydrolysis/acidogenesis pretreatment. Bioresour. Technol. 2012, 116, 441–447. [Google Scholar] [CrossRef]
- Aragon, C.; Quiroga, J.M.; Coello, M.D. Comparison of four chemical uncouplers for excess sludge reduction. Environ. Technol. 2009, 30, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Da, Z.; Feng, Y.; Wang, Y.; Cai, L.; Cui, H. Enhancing the electricity generation and sludge reduction of sludge microbial fuel cell with graphene oxide and reduced graphene oxide. J. Clean. Prod. 2018, 186, 104–112. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Li, B.; Feng, Y. Integrating sludge microbial fuel cell with inclined plate settling and membrane filtration for electricity generation, efficient sludge reduction and high wastewater quality. Chem. Eng. J. 2018, 331, 152–160. [Google Scholar] [CrossRef]
- Ali, J.; Wang, L.; Waseem, H.; Song, B.; Djellabi, R.; Pan, G. Turning harmful algal biomass to electricity by microbial fuel cell: A sustainable approach for waste management. Environ. Pollut. 2020, 266, 115373. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Narindri, B.; Chu, H.; Whang, L.-M. Recent advancement on biological technologies and strategies for resource recovery from swine wastewater. Bioresour. Technol. 2020, 303, 122861. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, J.; Liang, P.; Huang, X. Microbial community analysis in biocathode microbial fuel cells packed with different materials. Amb. Express 2012, 2, 21. [Google Scholar] [CrossRef]
- Moqsud, M.A. Bioelectricity generation and remediation of sulfide contaminated tidal flat sediment. Int. J. Sediment Res. 2020, 35, 91–96. [Google Scholar] [CrossRef]
- Wang, D.-B.; Song, T.-S.; Guo, T.; Zeng, Q.; Xie, J. Electricity generation from sediment microbial fuel cells with algae-assisted cathodes. Int. J. Hydrogen Energy 2014, 39, 13224–13230. [Google Scholar] [CrossRef]
- He, Z.; Shao, H.; Angenent, L.T. Increased power production from a sediment microbial fuel cell with a rotating cathode. Biosens. Bioelectron. 2007, 22, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, W.; Cao, X.; Wang, Y.; Zou, L.; Ge, X.; Zhao, Y.; Si, Z.; Wang, Y. Chlorella vulgaris on the cathode promoted the performance of sediment microbial fuel cells for electrogenesis and pollutant removal. Sci. Total Environ. 2020, 728, 138011. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, B.; Zhou, S.; Zhong, S.; Zhuang, L. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 2011, 102, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Rattaapha, W.; Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D.; Eaton, D.L.; Rice, W.; Greenberg, A.S.; Rice, E.W.; Connors, J.; Jenkis, D. Standards methods for the examination of water and wastewater. Health Lab. Sci. 1985, 4, 137. [Google Scholar]
- Yin, C.; Meng, F.; Chen, G.-H. Spectroscopic characterization of extracellular polymeric substances from a mixed culture dominated by ammonia-oxidizing bacteria. Water Res. 2015, 68, 740–749. [Google Scholar] [CrossRef]
- He, Q.; Wang, H.; Chen, L.; Gao, S.; Zhang, W.; Song, J.; Yu, J. Elevated salinity deteriorated enhanced biological phosphorus removal in an aerobic granular sludge sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal. J. Hazard. Mater. 2020, 390, 121782. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Z.; Yu, Z.; Dai, X.; Xu, X.; Alvarez, P.J.J.; Zhu, L. Evolution and functional analysis of extracellular polymeric substances during the granulation of aerobic sludge used to treat p-chloroaniline wastewater. Chem. Eng. J. 2017, 330, 596–604. [Google Scholar] [CrossRef]
- Touch, N.; Hibino, T.; Nagatsu, Y.; Tachiuchi, K. Characteristics of electricity generation and biodegradation in tidal river sludge-used microbial fuel cells. Bioresour. Technol. 2014, 158, 225–230. [Google Scholar] [CrossRef]
- Neethu, B.; Ghangrekar, M.M. Electricity generation through a photo sediment microbial fuel cell using algae at the cathode. Water Sci. Technol. 2017, 76, 3269–3277. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, F.; Grimaud, J.; Hurst, J.; He, Z. Long-term investigation of microbial fuel cells treating primary sludge or digested sludge. Bioresour. Technol. 2013, 136, 509–514. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, H.; Feng, Y.; Wang, Y.; Yu, M. Sludge decrement and electricity generation of sludge microbial fuel cell enhanced by zero valent iron. J. Clean. Prod. 2018, 174, 35–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Noori, J.S.; Angelidaki, I. Simultaneous organic carbon, nutrients removal and energy production in a photomicrobial fuel cell (PFC). Energy Environ. Sci. 2011, 4, 4340–4346. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Yu, H.; Yi, X.; Wei, C. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170. [Google Scholar] [CrossRef]
- Jiang, H.-m. Combination of Microbial Fuel Cells with Microalgae Cultivation for Bioelectricity Generation and Domestic Wastewater Treatment. Environ. Eng. Sci. 2017, 34, 489–495. [Google Scholar] [CrossRef]
- Liu, W.; Huang, W.; Cao, Z.; Ji, Y.; Liu, D.; Huang, W.; Zhu, Y.; Lei, Z. Microalgae simultaneously promote antibiotic removal and antibiotic resistance genes/bacteria attenuation in algal-bacterial granular sludge system. J. Hazard. Mater. 2022, 438, 129286. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, H.; Li, Q.; Zou, J.; Liu, M. Research On the Characteristics of Sediment and the Release Law of Nitrogen and Phosphorus Pollutants in Landscape Lake. J. Phys. Conf. Ser. 2022, 2186, 012026. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, Y.; Chen, Y.; Shen, N.; Wang, G.; Yan, Y. Realignment of phosphorus in lake sediment induced by sediment microbial fuel cells (SMFC). Chemosphere 2022, 291, 132927. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, X.; Gao, W.; Zhao, S.; Zhang, W.; Yu, P.; Wang, D. Malodorous volatile organic compounds (MVOCs) formation after dewatering of wastewater sludge: Correlation with the extracellular polymeric substances (EPS) and microbial communities. Sci. Total Environ. 2023, 867, 161491. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Liu, F.; Gao, M.; Wang, J.; Zhang, A.; Liu, Y. Impact of Al-based coagulants on the formation of aerobic granules: Comparison between poly aluminum chloride (PAC) and aluminum sulfate (AS). Sci. Total Environ. 2019, 685, 74–84. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dulski, T.; Ciesielski, S.; Thornton, A. Denitrification aided by waste beer in anaerobic sequencing batch biofilm reactor (AnSBBR). Ecol. Eng. 2016, 95, 384–389. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Z.; Zhang, P.; Li, X.; Feng, L. Polycyclic aromatic hydrocarbons stimulate acidogenesis, acetogenesis and methanogenesis during anaerobic co-digestion of waste activated sludge and food waste. Bioresour. Technol. 2022, 360, 127567. [Google Scholar] [CrossRef] [PubMed]
- Abrevaya, X.C.; Sacco, N.; Mauas, P.J.D.; Corton, E. Archaea-based microbial fuel cell operating at high ionic strength conditions. Extremophiles 2011, 15, 633–642. [Google Scholar] [CrossRef] [PubMed]









| Samples | Peak A | Peak B | Peak C | ||||
|---|---|---|---|---|---|---|---|
| Ex/Em | Intensity | Ex/Em | Intensity | Ex/Em | Intensity | ||
| LB-EPS | Initial | 270/340 | 345 | 225/328 | 239 | 325/434 | 63 |
| AC-SMFC | 280/334 | 90 | 240/330 | 105 | 330/420 | 34 | |
| BC-SMFC | 275/334 | 120 | 240/334 | 133 | 278/423 | 28 | |
| SS-system | 265/330 | 335 | 225/330 | 284 | 330/434 | 49 | |
| TB-EPS | Initial | 285/348 | 571 | 230/310 | 136 | 350/438 | 95 |
| AC-SMFC | 280/334 | 152 | 240/328 | 161 | - | - | |
| BC-SMFC | 280/334 | 139 | 240/328 | 152 | - | - | |
| SS-system | 275/340 | 433 | 240/320 | 284 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, H.; Li, Y.; Zhao, C.; Liu, L.; Li, L.; Sun, L.; Li, H. Sediment Microbial Fuel Cells with Algae-Assisted Cathodes for Electricity Generation and Bio-Treatment of Sewage Sludge. Fermentation 2023, 9, 1010. https://doi.org/10.3390/fermentation9121010
Chen L, Zhang H, Li Y, Zhao C, Liu L, Li L, Sun L, Li H. Sediment Microbial Fuel Cells with Algae-Assisted Cathodes for Electricity Generation and Bio-Treatment of Sewage Sludge. Fermentation. 2023; 9(12):1010. https://doi.org/10.3390/fermentation9121010
Chicago/Turabian StyleChen, Lizheng, Hongyi Zhang, Yongqi Li, Chunxia Zhao, Ling Liu, Lipin Li, Li Sun, and Hui Li. 2023. "Sediment Microbial Fuel Cells with Algae-Assisted Cathodes for Electricity Generation and Bio-Treatment of Sewage Sludge" Fermentation 9, no. 12: 1010. https://doi.org/10.3390/fermentation9121010