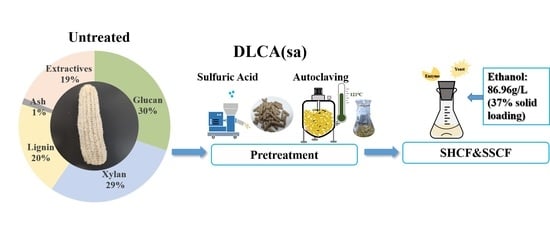
Efficient Corncob Biorefinery for Ethanol Initiated by a Novel Pretreatment of Densifying Lignocellulosic Biomass with Sulfuric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass and Cellulase
2.2. Pretreatment of Corncob by Densifying Biomass with Sulfuric Acid
2.3. Enzymatic Hydrolysis
2.4. Separate Hydrolysis and Co-Fermentation
2.5. Simultaneous Saccharification and Co-Fermentation
2.6. Analysis Methods
3. Results and Discussion
3.1. Effects of Sulfuric Acid Dosage, Solid Loading and Autoclave Time on Hydrolysis
3.2. Component Analysis and FT-IR/SEM
3.3. Enzymatic Hydrolysis of DLCA(sa)-CC at High Solids Loadings
3.4. Separate Hydrolysis and Co-Fermentation for Ethanol Production
3.5. Simultaneous Saccharification and Co-Fermentation for Ethanol Production
3.6. Mass Balance for SSCF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Razmjoo, A.A.; Sumper, A.; Davarpanah, A. Development of sustainable energy indexes by the utilization of new indicators: A comparative study. Energy Rep. 2019, 5, 375–383. [Google Scholar] [CrossRef]
- Rossetti, I.; Tripodi, A.; Ramis, G. Hydrogen, ethylene and power production from bioethanol: Ready for the renewable market? Int. J. Hydrogen Energ. 2020, 45, 10292–10303. [Google Scholar] [CrossRef]
- Kumar, M.N.; Ravikumar, R.; Thenmozhi, S.; Kumar, M.R.; Shankar, M.K. Choice of pretreatment technology for sustainable production of bioethanol from lignocellulosic biomass: Bottle necks and recommendations. Waste Biomass Valori. 2018, 10, 1693–1709. [Google Scholar] [CrossRef]
- Domínguez-Gómez, C.X.; Nochebuena-Morando, L.E.; Aguilar-Uscanga, M.G.; López-Zamora, L. Statistical optimization of dilute acid and H2O2 alkaline pretreatment using surface response methodology and tween 80 for the enhancement of the enzymatic hydrolysis of corncob. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Takada, M.; Niu, R.; Minami, E.; Saka, S. Characterization of three tissue fractions in corn (Zea mays) cob. Biomass Bioenergy 2018, 115, 130–135. [Google Scholar] [CrossRef]
- Morales, A.; Gullón, B.; Dávila, I.; Eibes, G.; Labidi, J.; Gullón, P. Optimization of alkaline pretreatment for the co-production of biopolymer lignin and bioethanol from chestnut shells following a biorefinery approach. Ind. Crops Prod. 2018, 124, 582–592. [Google Scholar] [CrossRef]
- Hoang, T.-D.; Nghiem, N. Recent Developments and current status of commercial production of fuel ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of various pretreatment methods on bioethanol production from cotton stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Sun, Z.-F.; Zhang, C.-C.; Nan, J.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Wang, G.S.; Lee, J.W.; Zhu, J.Y.; Jeffries, T.W. Dilute Acid pretreatment of corncob for efficient sugar production. Appl. Biochem. Biotechnol. 2011, 163, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Carrier, D.J. Understanding the Pine Dilute acid pretreatment system for enhanced enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2015, 3, 2423–2428. [Google Scholar] [CrossRef]
- Miyamoto, R.Y.; José, A.H.M.; Lopes, M.M.; Rodrigues, R.C.L.B. Effectiveness of baffled flasks on the growth of Scheffersomyces stipitis CBS 6054 inoculum for ethanol production in corncob hemicellulosic hydrolysate. Ind. Biotechnol. 2020, 16, 309–317. [Google Scholar] [CrossRef]
- Weiss, N.D.; Felby, C.; Thygesen, L.G. Enzymatic hydrolysis is limited by biomass-water interactions at high-solids: Improved performance through substrate modifications. Biotechnol. Biofuels 2019, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yuan, X.; Chen, S.; Yu, J.; Zhai, R.; Xu, Z.; Jin, M. Densifying Lignocellulosic biomass with alkaline Chemicals (DLC) pretreatment unlocks highly fermentable sugars for bioethanol production from corn stover. Green Chem. 2021, 23, 4828–4839. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, X.; Shen, G.; Chen, S.; Yu, J.; Zhai, R.; Xu, Z.; Jin, M. Densifying lignocellulosic biomass with sulfuric acid provides a durable feedstock with high digestibility and high fermentability for cellulosic ethanol production. Renew. Energy 2022, 182, 377–389. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, G.; Chen, S.; Chen, X.; Zhang, C.; Liu, S.; Jin, M. Modified simultaneous saccharification and co-fermentation of DLC pretreated corn stover for high-titer cellulosic ethanol production without water washing or detoxifying pretreated biomass. Energy 2022, 247, 123488. [Google Scholar] [CrossRef]
- Shen, G.; Yuan, X.; Chen, S.; Liu, S.; Jin, M. High titer cellulosic ethanol production from sugarcane bagasse via DLCA pretreatment and process development without washing/detoxifying pretreated biomass. Renew. Energy 2022, 186, 904–913. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Lam, F.H.; Ghaderi, A.; Fink, G.R.; Stephanopoulos, G. Engineering alcohol tolerance in yeast. Science 2014, 346, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Hu, J.; Saddler, J.N. Extent of Enzyme Inhibition by Phenolics Derived from pretreated biomass is significantly influenced by the size and carbonyl group content of the phenolics. ACS Sustain. Chem. Eng. 2018, 6, 3823–3829. [Google Scholar] [CrossRef]
- Uppugundla, N.; Sousa, L.D.C.; Chundawat, S.P.; Yu, X.; Simmons, B.; Singh, S.; Gao, X.; Kumar, R.; E Wyman, C.; E Dale, B.; et al. A comparative study of ethanol production using dilute acid, ionic liquid and AFEX (TM) pretreated corn stover. Biotechnol. Biofuels 2014, 7, 72. [Google Scholar] [CrossRef]
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-production of bioethanol and xylosaccharides from steam-exploded eucalyptus sawdust using high solid loads in enzymatic hydrolysis: Effect of alkaline impregnation. Ind. Crops Prod. 2022, 175, 114253. [Google Scholar] [CrossRef]
- Anukam, A.I.; Goso, B.P.; Okoh, O.O.; Mamphweli, S.N. Studies on Characterization of corn cob for application in a gasification process for energy production. J. Chem. 2017, 2017, 16685–16701. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Fu, J.; Chen, Z.; Ren, L. The effect of microstructure on mechanical properties of corn cob. Micron 2021, 146, 103070. [Google Scholar] [CrossRef] [PubMed]
- Na, B.-I.; Chang, S.-J.; Lee, K.H.; Lee, G.; Lee, J.-W. Characterization of cell wall structure in dilute acid-pretreated biomass by confocal Raman microscopy and enzymatic hydrolysis. Biomass Bioenergy 2016, 93, 33–37. [Google Scholar] [CrossRef]
- Zhang, J.; Viikari, L. Xylo-oligosaccharides are competitive inhibitors of cellobiohydrolase I from thermoascus aurantiacus. Bioresour. Technol. 2012, 117, 286–291. [Google Scholar] [CrossRef]
- Jonsson, L.J.; Martin, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment—A review. Biotechnol. Bioeng. 2012, 109, 1430–1442. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.-C.; Wang, Z.-J.; Ren, G.-H.; Kong, W.; Li, L.; Xie, W.; Liu, Y.-H. Engineering a novel glucose-tolerant beta-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol. Biofuels 2015, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Li, S.; Huang, X.; Qin, Z.; Kong, W.; Xie, W.; Liu, Y. Enhancing the thermostability of highly active and glucose-tolerant beta-Glucosidase Ks5A7 by directed evolution for good performance of three properties. J. Agric. Food Chem. 2018, 66, 13228–13235. [Google Scholar] [CrossRef]
- Rajasree, K.P.; Mathew, G.M.; Pandey, A.; Sukumaran, R.K. Highly glucose tolerant beta-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J. Ind. Microbiol. Biotechnol. 2013, 40, 967–975. [Google Scholar] [CrossRef]
- Bondesson, P.M.; Galbe, M. Process design of SSCF for ethanol production from steam-pretreated, acetic-acid-impregnated wheat straw. Biotechnol. Biofuels 2016, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Rahikainen, J.L.; Martin-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Rojas, O.J.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef]
- Roberts, K.M.; Lavenson, D.M.; Tozzi, E.J.; McCarthy, M.J.; Jeoh, T. The effects of water interactions in cellulose suspensions on mass transfer and saccharification efficiency at high solids loadings. Cellulose 2011, 18, 759–773. [Google Scholar] [CrossRef]
- Gupta, R.; Mehta, G.; Kuhad, R.C. Fermentation of pentose and hexose sugars from corncob, a low cost feedstock into ethanol. Biomass Bioenergy 2012, 47, 334–341. [Google Scholar] [CrossRef]
- Selvakumar, P.; Adane, A.; Zelalem, T.; Hunegnaw, B.; Karthik, V.; Kavitha, S.; Jayakumar, M.; Karmegam, N.; Govarthanan, M.; Kim, W. Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel 2022, 321, 124060. [Google Scholar] [CrossRef]
- Sunkar, B.; Bhukya, B. Bi-phasic hydrolysis of corncobs for the extraction of total sugars and ethanol production using inhibitor resistant and thermotolerant yeast, Pichia kudriavzevii. Biomass Bioenergy 2021, 153, 106033. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Chen, Y.; Fu, G.; Li, B.; Guo, X.; Xiao, D. Efficient utilization of hemicellulose and cellulose in alkali liquor-pretreated corncob for bioethanol production at high solid loading by Spathaspora passalidarum U1-58. Bioresour. Technol. 2017, 232, 168–175. [Google Scholar] [CrossRef]
- Ismail, K.S.K.; Matano, Y.; Sakihama, Y.; Inokuma, K.; Nambu, Y.; Hasunuma, T.; Kondo, A. Pretreatment of extruded Napier grass byhydrothermal process with dilute sulfuric acid and fermentation using a cellulose-hydrolyzing and xylose-assimilating yeast for ethanol production. Bioresour. Technol. 2022, 343, 126071. [Google Scholar] [CrossRef]
- Su, R.; Ma, Y.; Qi, W.; Zhang, M.; Wang, F.; Du, R.; Yang, J.; Zhang, M.; He, Z. Ethanol production from high-solid SSCF of alkaline-pretreated corncob using recombinant Zymomonas mobilis CP4. Bioenerg. Res. 2013, 6, 292–299. [Google Scholar] [CrossRef]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Sharma, G.D.; Ranjitha, J.; Gupta, V.K. Second-generation bioethanol production from corncob – A comprehensive review on pretreatment and bioconversion strategies, including techno-economic and lifecycle perspective. Ind. Crops Prod. 2022, 186, 115245. [Google Scholar] [CrossRef]
- Brar, K.K.; Kaur, S.; Chadha, B.S. A novel staggered hybrid SSF approach for efficient conversion of cellulose/hemicellulosic fractions of corncob into ethanol. Renew. Energy 2016, 98, 16–22. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Inokuma, K.; Johansson, B.; Hasunuma, T.; Kondo, A.; Domingues, L. Consolidated bioprocessing of corn cob-derived hemicellulose: Engineered industrial Saccharomyces cerevisiae as efficient whole cell biocatalysts. Biotechnol. Biofuels 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lan, M.; Gao, X.; Dou, Y.; Zhang, X. Sequential dilute acid/ alkali pretreatment of corncobs for ethanol production. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 43, 1769–1778. [Google Scholar] [CrossRef]









| Frequency Range (cm−1) | Functional Groups | Class of Compounds |
|---|---|---|
| 890 | C-H bending | Aromatic compounds in cellulose |
| 1053 | C-O stretching | Alcohol, phenols and esters in cellulose |
| 1245 | -OH bending | Phenolic and aliphatic structures in cellulose |
| 1332 | C-C and C-O stretching | Acetyl groups in lignin |
| 1385 | C-H bending | Aliphatic structures in cellulose and hemicellulose |
| 1420 | C-C stretching | Benzene rings in lignin |
| 1520 | C=C bending | Aromatic compounds in lignin |
| 1608 | C=O stretching | Carbonyl bonds in lignin |
| 1727 | C=O stretching | Carboxylic acids/ester groups in hemicellulose |
| 2893 | C-H stretching | Alkanes in cellulose |
| 3362 | -OH descending | Alcohol, phenols in cellulose |
| Solid Loading | Sugar Conversion (%) | Sugar Consumption (%) | Ethanol Yield (%) | Ethanol Titer (g/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Glucan | Xylan | Total Sugar | Glucan | Xylan | Total Sugar | ||||
| SHCF | 25% | 95.6 ± 0.4 | 94.1 ± 1.3 | 94.9 ± 0.9 | 100 ± 0.0 | 96.1 ± 0.05 | 98.3 ± 0.02 | 85.1 ± 0.4 | 66.3 ± 0.3 |
| 30% | 97.3 ± 0.1 | 97.8 ± 0.8 | 97.6 ± 0.5 | 100 ± 0.0 | 84.5 ± 0.4 | 93.3 ± 0.2 | 85.1 ± 0.8 | 73.5 ± 0.8 | |
| 32% | 96.2 ± 0.5 | 93.5 ± 1.6 | 94.9 ± 1.1 | 100 ± 0.0 | 82.1 ± 0.4 | 92.3 ± 0.3 | 81.6 ± 0.6 | 75.2 ± 0.7 | |
| 35% | 94.0 ± 0.3 | 91.5 ± 0.2 | 92.8 ± 0.3 | 100 ± 0.0 | 70.2 ± 0.4 | 87.2 ± 0.2 | 80.1 ± 0.04 | 75.7 ± 0.05 | |
| SSCF | 25% | 91.5 ± 0.02 | 97.0 ± 0.04 | 94.1 ± 0.03 | 100 ± 0.0 | 96.2 ± 0.03 | 98.3 ± 0.02 | 92.2 ± 0.8 | 63.6 ± 0.6 |
| 30% | 87.0 ± 1.2 | 96.2 ± 0.3 | 91.3 ± 0.7 | 98.8 ± 0.1 | 96.2 ± 0.04 | 97.5 ± 0.07 | 90.9 ± 0.8 | 74.0 ± 0.8 | |
| 32% | 81.9 ± 4.5 | 94.9 ± 1.2 | 88.0 ± 3.0 | 98.7 ± 0.4 | 95.4 ± 0.04 | 96.9 ± 0.3 | 91.9 ± 0.2 | 78.1 ± 0.2 | |
| 35% | 80.4 ± 0.9 | 94.4 ± 0.2 | 86.9 ± 0.6 | 99.9 ± 0.03 | 90.0 ± 1.3 | 94.7 ± 0.9 | 94.5 ± 0.01 | 82.0 ± 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yu, Y.; Xu, Z.; Chen, S.; Shen, G.; Yuan, X.; Deng, Q.; Shen, W.; Yang, S.; Zhang, C.; et al. Efficient Corncob Biorefinery for Ethanol Initiated by a Novel Pretreatment of Densifying Lignocellulosic Biomass with Sulfuric Acid. Fermentation 2022, 8, 661. https://doi.org/10.3390/fermentation8110661
Liu S, Yu Y, Xu Z, Chen S, Shen G, Yuan X, Deng Q, Shen W, Yang S, Zhang C, et al. Efficient Corncob Biorefinery for Ethanol Initiated by a Novel Pretreatment of Densifying Lignocellulosic Biomass with Sulfuric Acid. Fermentation. 2022; 8(11):661. https://doi.org/10.3390/fermentation8110661
Chicago/Turabian StyleLiu, Shuangmei, Yang Yu, Zhaoxian Xu, Sitong Chen, Guannan Shen, Xinchuan Yuan, Qiufeng Deng, Wenyuan Shen, Shizhong Yang, Chengcheng Zhang, and et al. 2022. "Efficient Corncob Biorefinery for Ethanol Initiated by a Novel Pretreatment of Densifying Lignocellulosic Biomass with Sulfuric Acid" Fermentation 8, no. 11: 661. https://doi.org/10.3390/fermentation8110661