Waste Apple Pomace Conversion to Acrylic Acid: Economic and Potential Environmental Impact Assessments
Abstract
:1. Introduction
2. Materials and Methods
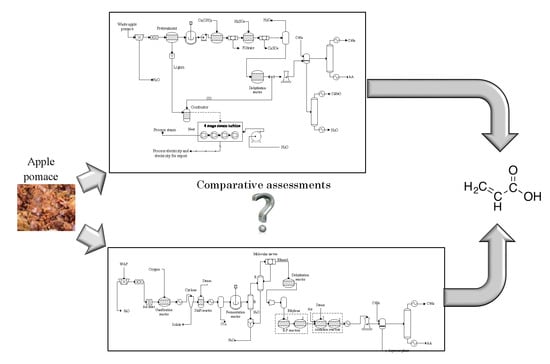
2.1. Process Description
2.2. Economic Assessments
2.3. Environmental Performance Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FD | Fermentation-dehydration |
| TFO | Thermochemical-fermentation-oxidation |
| WAP | Waste apple pomace |
| AA | Acrylic acid |
| LA | Lactic acid |
| GHG | Greenhouse gas |
| NRTL | Non-random two-liquid |
| RSTOIC | Stoichiometric reactor model |
| RGIBBS | Equilibrium reactor model |
| RADFRAC | Rigorous distillation column model |
| CHP | Combined heat and power |
| HHV | higher heating value |
| TCI | total capital investment |
| TOC | total operating costs |
| FCI | Fixed capital investment |
| WCI | Working capital investment |
| TDC | Total direct cost |
| TIDC | Total indirect cost |
| APEA | Aspen Process Economic Analyzer |
| CEPCI | Chemical Engineering Plant Cost Index |
References
- Moriarty, P.; Honnery, D. The risk of catastrophic climate change: Future energy implications. Futures 2021, 128, 102728. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels—A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Liu, C.-G.; Reungsang, A. Editorial: Special issue “Sustainable biorefinery approaches”. Biomass Convers. Biorefinery 2021, 11, 741–742. [Google Scholar] [CrossRef]
- Yates, M.; Gomez, M.R.; Martin-Luengo, M.A.; Ibañez, V.Z.; Martinez Serrano, A.M. Multivalorization of apple pomace towards materials and chemicals. Waste to wealth. J. Clean. Prod. 2017, 143, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Okoro, O.V.; Shavandi, A. An assessment of the utilization of waste apple slurry in bio-succinic acid and bioenergy production. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Landi, G.; Lisi, L.; Russo, G. Oxidation of propane and propylene to acrylic acid over vanadyl pyrophosphate. J. Mol. Catal. A Chem. 2005, 239, 172–179. [Google Scholar] [CrossRef]
- Mazloom, G.; Alavi, S.M. Kinetic study of selective propane oxidation to acrylic acid over Mo1V0.3Te0.23Nb0.12Ox using the genetic algorithm. React. Kinet. Mech. Catal. 2013, 110, 387–403. [Google Scholar] [CrossRef]
- Scott, J. Acrylic Acid Production via Propylene Oxidation—By Intratec Solutions: Chemengonline. 2016. Available online: https://www.chemengonline.com/acrylic-acid-production-via-propylene-oxidation-intratec-solutions/ (accessed on 30 April 2021).
- Brown, D. Acrylic Acid. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 74–75. [Google Scholar]
- Serrano-Aroca, A. Latest improvements of acrylic-based polymer properties for biomedical applications. In Acrylic Polymers in Healthcare; InTechOpen: Rijeka, Croatia, 2017; Volume 1, p. 13. [Google Scholar]
- Jin, X.; Meng, K.; Zhang, G.; Liu, M.; Song, Y.; Song, Z.; Yang, C. Interfacial catalysts for sustainable chemistry: Advances on atom and energy efficient glycerol conversion to acrylic acid. Green Chem. 2021, 23, 51–76. [Google Scholar] [CrossRef]
- Staples, T.L.; Chatterjee, P.K. Chapter VIII—Synthetic Superabsorbents. In Textile Science and Technology; Chatterjee, P.K., Gupta, B.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 13, pp. 283–322. [Google Scholar]
- Grand-View-Research. Acrylic Acid Market Size Worth $22.55 Billion by 2022; Grand-View-Research: Dublin, Ireland, 2016. [Google Scholar]
- Expertmarketresearch. Global Acrylic Acid Market: By Applications: Acrylate Esters, Super Absorbent Polymer (SAP), Others; Regional Analysis; Historical Market and Forecast (2016–2026); Market Dynamics; SWOT Analysis; Porter’s Five Forces Analysis; Value Chain Analysis; Compet; Expertmarketresearch: Sheridan, WY, USA, 2020. [Google Scholar]
- Kim, M.; Lee, H. Highly Selective Production of Acrylic Acid from Glycerol via Two Steps Using Au/CeO2 Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 11371–11376. [Google Scholar] [CrossRef]
- Danner, H.; Ürmös, M.; Gartner, M.; Braun, R. Biotechnological production of acrylic acid from biomass. Appl. Biochem. Biotechnol. 1998, 70, 887–894. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Sie, S.; Franco, T.T.; van der Wielen, L.A.M. Feasibility of acrylic acid production by fermentation. Appl. Microbiol. Biotechnol. 2005, 67, 727–734. [Google Scholar] [CrossRef]
- Petrescu, L.; Fermeglia, M.; Cormos, C.-C. Life Cycle Analysis applied to acrylic acid production process with different fuels for steam generation. J. Clean. Prod. 2016, 133, 294–303. [Google Scholar] [CrossRef]
- Iglesias, J.; Martínez-Salazar, I.; Maireles-Torres, P.; Martin Alonso, D.; Mariscal, R.; López Granados, M. Advances in catalytic routes for the production of carboxylic acids from biomass: A step forward for sustainable polymers. Chem. Soc. Rev. 2020, 49, 5704–5771. [Google Scholar] [CrossRef]
- Yan, B.; Liu, Z.-H.; Liang, Y.; Xu, B.-Q. Acrylic Acid Production by Gas-Phase Dehydration of Lactic Acid over K+-Exchanged ZSM-5: Reaction Variable Effects, Kinetics, and New Evidence for Cooperative Acid–Base Bifunctional Catalysis. Ind. Eng. Chem. Res. 2020, 59, 17417–17428. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, T. Efficient production of acrylic acid by dehydration of lactic acid over BaSO4 with crystal defects. RSC Adv. 2017, 7, 10278–10286. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszak, R.; Bonnotte, T.; Paul, S.; Katryniok, B.; Dumeignil, F. Lactic Acid Conversion to Acrylic Acid Over Fluoride-Substituted Hydroxyapatites. Front. Chem. 2020, 8, 421. [Google Scholar] [CrossRef]
- De Resende, T.M.; da Costa, M.M. Biopolymers of sugarcane. In Sugarcane Biorefinery, Technology and Perspectives; Santos, F., Rabelo, S.C., De Matos, M., Eichler, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 229–254. [Google Scholar]
- Kehrer, M. Liquid-Phase Dehydration of Lactic Acid for the Production of Bio-Acrylic acid Development of a Multi-Step Process. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 17 December 2018. [Google Scholar]
- Xu, X.; Lin, J.; Cen, P. Advances in the Research and Development of Acrylic Acid Production from Biomass1 1Supported by the Special Funds for Major State Basic Research Program of China (973 Program, No.2004CCA05500). Chin. J. Chem. Eng. 2006, 14, 419–427. [Google Scholar] [CrossRef]
- Pistikopoulos, E.N.; Stefanis, S.K.; Livingston, A.G.A. Methodology for Minimum Environmental Impact Analysis. Symp. Ser. 1995, 35, 4128. [Google Scholar]
- Shonnard, D.R.; Hiew, D.S. Comparative Environmental assessments of VOC Recovery and recycle design alaternatives for gaseous waste streams. Environ. Sci. Technol. 2000, 34, 5222–5228. [Google Scholar] [CrossRef]
- Young, D.M.; Cabezas, H. Designing sustainable processes with simulation: The waste reduction (WAR) algorithm. Comput. Chem. Eng. 1999, 23, 1477–1491. [Google Scholar] [CrossRef]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of Bio-Based Films and 3D Objects from Apple Pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of Fruit Wastes: A Promising Technique for Generating Hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Marcotullio, G. The Chemistry and Technology of Furfural Production in Modern Lignocellulose-Feedstock Biorefineries. 2011. Available online: https://www.researchgate.net/publication/254908764_The_Chemistry_and_Technology_of_Furfural_Production_in_Modern_Lignocellulose-Feedstock_Biorefineries (accessed on 8 December 2021).
- Peña-Tejedor, S.; Murga, R.; Sanz, M.T.; Beltrán, S.J.F.P.E. Vapor–liquid equilibria and excess volumes of the binary systems ethanol+ ethyl lactate, isopropanol+ isopropyl lactate and n-butanol+ n-butyl lactate at 101.325 kPa. Fluid Phase Equilibria 2005, 230, 197–203. [Google Scholar] [CrossRef]
- Deterre, S.; Albet, J.; Joulia, X.; Baudouin, O.; Giampaoli, P.; Decloux, M.; Athes, V.J.J.O.C.; Data, E. Vapor–liquid equilibria measurements of bitter orange aroma compounds highly diluted in boiling hydro-alcoholic solutions at 101.3 kPa. J. Chem. Eng. Data 2012, 57, 3344–3356. [Google Scholar] [CrossRef]
- Vu, D.T.; Lira, C.T.; Asthana, N.S.; Kolah, A.K.; Miller, D.J.J.J.O.C.; Data, E. Vapor−liquid equilibria in the systems ethyl lactate+ ethanol and ethyl lactate+ water. J. Chem. Eng. Data 2006, 51, 1220–1225. [Google Scholar] [CrossRef]
- Cempa-Balewicz, M.; Jacek Łączny, M.; Smoliński, A.; Iwaszenko, S. Equilibrium Model of Steam Gasification of Coal. J. Sustain. Min. 2013, 12, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Thermo-Chemical Analysis of Biomass Gasification by Gibbs Free Energy Minimization Model-Part: II (Optimization of Biomass Feed and Steam to Biomass Ratio). Int. J. Green Energy 2013, 10, 610–639. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Okoro, O.V.; Sun, Z.; Birch, J. Techno-Economic Assessment of a Scaled-Up Meat Waste Biorefinery System: A Simulation Study. Materials 2019, 12, 1030. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.M.; Chireshe, F.; Okoro, O.; Gorgens, J.; Van Dyk, J. Evaluating refinery configurations for deriving sustainable aviation fuel from ethanol or syncrude. Fuel Process. Technol. 2021, 219, 106879. [Google Scholar] [CrossRef]
- ASPEN-plus. Aspen Plus User Guide; ASPEN Technology Incorporated: Cambridge, MA, USA, 2000. [Google Scholar]
- Petersen, A.M.; Okoro, O.V.; Chireshe, F.; Moonsamy, T.; Görgens, J.F. Systematic cost evaluations of biological and thermochemical processes for ethanol production from biomass residues and industrial off-gases. Energy Convers. Manag. 2021, 243, 114398. [Google Scholar] [CrossRef]
- Okoro, O.V.; Nie, L.; Hobbi, P.; Shavandi, A. Valorization of Waste Apple Pomace for Production of Platform Biochemicals: A Multi-Objective Optimization Study. Waste Biomass Valorization 2021, 12, 6887–6901. [Google Scholar] [CrossRef]
- Alibaba. Mechanical Dewatering Device. Available online: https://www.alibaba.com/product-detail/Dewatering-Dewatering-Machine-NORSEN-Vehicular-Sludge_1600051333912.html?spm=a2700.galleryofferlist.normal_offer.d_title.5c49383dKmOCni&s=p (accessed on 18 February 2021).
- Zimmer, E. Optimal Use of Resources and Energy During Fruit Juice Extraction. In Fruit Processing; Bucher Unipektin A: Nlederwenlngen, Switzerland, 2017. [Google Scholar]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, J.A.; Ballesteros, I.; Ballesteros, M.; Sáez, F.; Negro, M.J.; Manzanares, P. Optimizing Liquid Hot Water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel 2008, 87, 3640–3647. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Biobutanol production from apple pomace: The importance of pretreatment methods on the fermentability of lignocellulosic agro-food wastes. Appl. Microbiol. Biotechnol. 2017, 101, 8041–8052. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.V.; Thomas, S.; Lee, Y.Y. High-yield fermentation of pentoses into lactic acid. Appl. Biochem. Biotechnol. 2000, 84, 665–677. [Google Scholar] [CrossRef]
- Su, C.-Y.; Yu, C.-C.; Chien, I.L.; Ward, J.D. Control of Highly Interconnected Reactive Distillation Processes: Purification of Raw Lactic Acid by Esterification and Hydrolysis. Ind. Eng. Chem. Res. 2015, 54, 6932–6940. [Google Scholar] [CrossRef]
- Kamble, S.P.; Barve, P.P.; Joshi, J.B.; Rahman, I.; Kulkarni, B.D. Purification of Lactic Acid via Esterification of Lactic Acid Using a Packed Column, Followed by Hydrolysis of Methyl Lactate Using Three Continuously Stirred Tank Reactors (CSTRs) in Series: A Continuous Pilot Plant Study. Ind. Eng. Chem. Res. 2012, 51, 1506–1514. [Google Scholar] [CrossRef]
- Lee, H. Development of Lactic and Succinic Acid Biorefinery Configurations for Integration into a Thermomechanical Pulp Mill. Master’s Thesis, École Polytechnique de Montréal, Montreal, QC, Canada, 2015. [Google Scholar]
- Ghantani, V.C.; Lomate, S.T.; Dongare, M.K.; Umbarkar, S.B. Catalytic dehydration of lactic acid to acrylic acid using calcium hydroxyapatite catalysts. Green Chem. 2013, 15, 1211–1217. [Google Scholar] [CrossRef]
- Engineeringtoolbox. Fuel Gases Heating Values. Engineeringtoolbox. 2021. Available online: https://www.engineeringtoolbox.com/heating-values-fuel-gases-d_823.html (accessed on 24 April 2021).
- Berg, L. Dehydration of Acrylic Acid by Extractive Distillation. U.S. Patent 5,154,800, 13 October 1992. [Google Scholar]
- Song, D.; Yang, J.-H.; Lee, C.-J. Conceptual design of water separation process in glycerol-based acrylic acid production. Chem. Eng. Res. Des. 2020, 156, 324–332. [Google Scholar] [CrossRef]
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an Equilibrium-based Model of Gasification of Biomass by Aspen Plus. Energy Procedia 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- Pardo-Planas, O.; Atiyeh, H.K.; Phillips, J.R.; Aichele, C.P.; Mohammad, S. Process simulation of ethanol production from biomass gasification and syngas fermentation. Bioresour. Technol. 2017, 245, 925–932. [Google Scholar] [CrossRef]
- Okoro, O.V.; Faloye, F.D. Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies. AgriEngeneering 2020, 2, 378–392. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Acharya, B.; Heidari, M.; Al-Salem, S.M.; Dutta, A. Hydrogen-Rich Gas Stream from Steam Gasification of Biomass: Eggshell as a CO2 Sorbent. Energy Fuels 2020, 34, 4828–4836. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Performance prediction of steam gasification of wood using an ASPEN PLUS thermodynamic equilibrium model. Int. J. Sustain. Energy 2014, 33, 416–434. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D. Aspen plus simulation of biomass gasification in a steam blown dual fluidised bed. In Materials and Processes for Energy: Communicating Current Research and Technological Developments; Formatex Research Centre: Norristown, PA, USA, 2013. [Google Scholar]
- Basu, P. (Ed.) Chapter 9—Production of Synthetic Fuels and Chemicals from Biomass. In Biomass Gasification and Pyrolysis; Academic Press: Cambridge, MA, USA, 2010; pp. 301–323. [Google Scholar]
- Ukpong, M.N.; Atiyeh, H.K.; De Lorme, M.J.; Liu, K.; Zhu, X.; Tanner, R.S.; Wilkins, M.R.; Stevenson, B.S. Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol. Bioeng. 2012, 109, 2720–2728. [Google Scholar] [CrossRef]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int. J. Syst. Evol. Microbiol. 1993, 43, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.R.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. Biological production of ethanol from coal synthesis gas. Appl. Biochem. Biotechnol. 1993, 39, 559–571. [Google Scholar] [CrossRef]
- Petersen, A.M.; Okoro, O.V.; Du Preez, J.; Görgens, J.F. Evaluation of Biorefining Scenarios for Advanced Fuels Production from Triticale Grain. Energy Fuels 2020, 34, 11003–11013. [Google Scholar] [CrossRef]
- Arvidsson, M.; Lundin, B. Process Integration Study of a Biorefinery Producing Ethylene from Lignocellulosic Feedstock for a Chemical Cluster. Master’s Thesis, Chalmers University of Technology, Goteborg, Sweden, 2011. [Google Scholar]
- Calvar, N.; González, B.; Dominguez, A. Esterification of acetic acid with ethanol: Reaction kinetics and operation in a packed bed reactive distillation column. Chem. Eng. Process Intensif. 2007, 46, 1317–1323. [Google Scholar] [CrossRef]
- Beucher, R.; Cammarano, C.; Rodríguez-Castellón, E.; Hulea, V. Direct conversion of ethylene to propylene over Ni- and W-based catalysts: An unprecedented behaviour. Catal. Commun. 2020, 144, 106091. [Google Scholar] [CrossRef]
- Pérez Sánchez, A.; Pérez Sánchez, E.J.; Segura Silva, R.M. Simulation of the acrylic acid production process through catalytic oxidation of gaseous propylene using ChemCAD® simulator. J. Ingeniare Revista Chilena Ingeniería 2019, 27, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Wibawanta, S.A.S. Catalytic Partial Oxidation of Propylene for Acrolein Production. Master’s Thesis, Curtin University, Perth, Australia, March 2011. [Google Scholar]
- Kanidarapu, N.R.; Reddy, G.K.; Prasad, P.R.; Sujatha, V. Design and Pinch Analysis of Methyl Acetate Production Process using Aspen Plus and Aspen Energy Analyzer. Int. J. Chem. Eng. Process. 2015, 1, 31–40. [Google Scholar]
- Okoro, O.V.; Nkrumah Banson, A.; Zhang, H. Circumventing Unintended Impacts of Waste N95 Facemask Generated during the COVID-19 Pandemic: A Conceptual Design Approach. Chem. Eng. 2020, 4, 54. [Google Scholar] [CrossRef]
- Devi, A.; Niazi, A.; Ramteke, M.; Upadhyayula, S. Techno-economic analysis of ethanol production from lignocellulosic biomass–a comparison of fermentation, thermo catalytic, and chemocatalytic technologies. Bioprocess Biosyst. Eng. 2021, 44, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Sinnot, R.; Towler, G. Chemical Engineering Design; Elsevier: Burlington, NJ, USA, 2009. [Google Scholar]
- Lamidi, R.O.; Wang, Y.; Pathare, P.B.; Roskilly, A.P.; Aguilar, M.C. Biogas Tri-generation for Postharvest Processing of Agricultural Products in a Rural Community: Techno-economic Perspectives. Energy Procedia 2017, 142, 63–69. [Google Scholar] [CrossRef]
- Granjo, J.; Duarte, B.; Oliveira, N. Soybean biorefinery: Process simulation and analysis. J. Chem. Eng. Trans. 2015, 45, 583–588. [Google Scholar]
- Dimitriou, I.; García-Gutiérrez, P.; Elder, R.H.; Cuéllar-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Talmadge, M.; Hensley, J.; Worley, M.; Dudgeon, D.; Barton, D.; Groendijk, P.; Ferrari, D.; Stears, B.; Searcy, E.M. Process Design and Economics for Conversion of Lignocellulosic Biomass to Ethanol: Thermochemical Pathway by Indirect Gasification and Mixed Alcohol Synthesis; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2011. [Google Scholar]
- Rickman, W.S. Handbook of Incineration of Hazardous Wastes (1991); CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Towler, G.; Sinnott, R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Spath, P.; Aden, A.; Eggeman, T.; Ringer, M.; Wallace, B.; Jechura, J. Biomass to Hydrogen Production Detailed Design and Economics Utilizing the Battelle Columbus Laboratory Indirectly-Heated Gasifier; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- DOE. Cost Estimating Guide; Department of Energy: Washington, DC, USA, 2018; Available online: https://www.directives.doe.gov/directives-documents/400-series/0413.3-EGuide-21A/@@images/file (accessed on 10 September 2021).
- Young, D.; Scharp, R.; Cabezas, H. The waste reduction (WAR) algorithm: Environmental impacts, energy consumption, and engineering economics. Waste Manag. 2000, 20, 605–615. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Catalyst-Free Biodiesel Production Methods: A Comparative Technical and Environmental Evaluation. Sustainability 2018, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, H.; Bare, J.; Mallick, S. Pollution prevention with chemical process simulators: The generalized waste reduction (WAR) algorithm–full version. Comput. Chem. Eng. 1999, 23, 623–634. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Pubchem. Acrylic Acid. National Library of Medicine. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acrylic-acid (accessed on 10 October 2021).
- WHO. Acrylic Acid Health and Safety Guide; IPCS International Programme on Chemical Safety Health and Safety Guide No. 104; WHO: Geneva, Switzerland, 1997. [Google Scholar]
- Culp, A.; Holmes, K.; Nagrath, R.; Nessenson, D. Propane to Acrylic Acid; School of Engineering and Applied Science, University of Pennsylvania: Philadelphia, PA, USA, 2013. [Google Scholar]
- Salaudeen, S.A.; Acharya, B.; Dutta, A. Steam gasification of hydrochar derived from hydrothermal carbonization of fruit wastes. Renew. Energy 2021, 171, 582–591. [Google Scholar] [CrossRef]
- Xia, W.; Takahashi, A.; Nakamura, I.; Shimada, H.; Fujitani, T. Study of active sites on the MFI zeolite catalysts for the transformation of ethanol into propylene. J. Mol. Catal. A Chem. 2010, 328, 114–118. [Google Scholar] [CrossRef]
- Gullón, B.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Production of l-lactic Acid and Oligomeric Compounds from Apple Pomace by Simultaneous Saccharification and Fermentation. A Response Surface Methodology Assessment. J. Agric. Food Chem. 2007, 55, 5580–5587. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Midgett, J.S. Assessing a Hydrothermal Liquefaction Process Using Biomass Feedstocks. Master’s Thesis, Luisiana State University, Baton Rouge, LA, USA, May 2008. [Google Scholar]
- Remón, J.; Randall, J.; Budarin, V.L.; Clark, J.H. Production of bio-fuels and chemicals by microwave-assisted, catalytic, hydrothermal liquefaction (MAC-HTL) of a mixture of pine and spruce biomass. Green Chem. 2019, 21, 284–299. [Google Scholar] [CrossRef]
- Demirbas, A. Higher heating values of lignin types from wood and non-wood lignocellulosic biomasses. Energy Sources Part A: Recovery Util. Environ. Eff. 2017, 39, 592–598. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing dissolved air flotation sludge as a potential biodiesel feedstock in New Zealand: A predictive analysis of the biodiesel product properties. J. Clean. Prod. 2017, 168, 1436–1447. [Google Scholar] [CrossRef]
- Cheali, P.; Gernaey, K.V.; Sin, G. Uncertainties in Early-Stage Capital Cost Estimation of Process Design—A Case Study on Biorefinery Design. Front. Energy Res. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Alibaba. Premium Industrial Grade Glacial Acrylic Acid 79-10-7 with Purity 99.9% Min Acrylic Acid Liquid for Water Treatment. 2021. Available online: https://www.alibaba.com/product-detail/Premium-Industrial-Grade-Glacial-Acrylic-Acid_1600360477213.html?spm=a2700.galleryofferlist.normal_offer.d_title.68a62f5dV4rcRI (accessed on 13 September 2021).
- Bhagwat, S.S.; Li, Y.; Cortés-Peña, Y.R.; Brace, E.C.; Martin, T.A.; Zhao, H.; Guest, J.S. Sustainable Production of Acrylic Acid via 3-Hydroxypropionic Acid from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 16659–16669. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Base year | 2020 |
| Project lifetime (y) | 30 |
| Plant availability (h/y) | 7200 |
| Tax rate (%) | 30 |
| Discount rate (%) | 10 |
| Salvage value (USD) | 0 |
| Depreciation | Straight line |
| General Impact Category | Impact Category | Measure of Impact Category |
|---|---|---|
| Human toxicity | Ingestion (HTPI) | LD50 |
| Inhalation/dermal (HTPE) | OSHA PEL | |
| Ecological toxicity | Aquatic toxicity (ATP) | Fathead minnow LC50 |
| Terrestrial toxicity (TTP) | LD50 | |
| Global atmospheric impacts | Global warming potential (GWP) | GWP |
| Ozone depletion potential (ODP) | ODP | |
| Regional atmospheric impacts | Acidification potential (AP) | AP |
| Photochemical oxidation potential (PCOP) | PCOP |
| Stream Name | FD Process | TFO Process | ||||||
|---|---|---|---|---|---|---|---|---|
| Hydrolysate | Stream LA | AA | AC | Syngas | EA | Stream P | AA | |
| Mass fractions (x) | ||||||||
| 2-Butene | 0 | 0 | 0 | 0 | 0 | 0 | 0.550 | 0 |
| AC | 0 | 0 | 0 | 0.980 | 0 | 0 | 0 | 0 |
| AA | 0 | 0 | 0.970 | 0 | 0 | 0 | 0 | 0.970 |
| Carbon dioxide | 0 | 0 | 0 | 0 | 0.610 | 0 | 0 | 0 |
| Carbon monoxide | 0 | 0 | 0 | 0 | 0.310 | 0 | 0 | 0 |
| Ethanol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fructose | 0.018 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glucose | 0.024 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydrogen | 0 | 0 | 0 | 0 | 0.080 | 0 | 0 | 0 |
| LA | 0 | 0.500 | 0 | 0 | 0 | 0 | 0 | 0 |
| Methane | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Propylene | 0 | 0 | 0 | 0 | 0 | 0 | 0.450 | 0 |
| Toluene | 0 | 0 | 0.030 | 0 | 0 | 0 | 0 | 0.030 |
| EA | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Water | 0.952 | 0.500 | 0 | 0.020 | 0 | 0 | 0 | 0 |
| Xylose | 0.006 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mass flows (tons/h) | 99.30 | 7.83 | 1.94 | 0.33 | 0.483 | 1.166 | 0.478 | 0.313 |
| Process Result | FD Process | TFO Process |
|---|---|---|
| Externally required heating utility (MW) | 59.66 | - |
| Externally required cooling utility (MW) | 27.48 | 15.87 |
| Net electricity generated (MW) | 11.45 | 0.83 |
| Costing Components | FD Process | TFO Process |
|---|---|---|
| Warehouse cost (USD) | 342,807.9 | 536,738.2 |
| Equipment installation cost (USD) | 771,317.8 | 1,207,661.0 |
| Home office and construction fee (USD) | 385,658.9 | 603,830.5 |
| Site development (USD) | 8,570,198.2 | 13,418,455.1 |
| Prorateable expenses (USD) | 1,006,998.3 | 1,576,668.5 |
| Project contingency (USD) | 1,006,998.3 | 1,576,668.5 |
| Additional piping (USD) | 2,013,996.6 | 3,153,337.0 |
| Field expenses (USD) | 1,006,998.3 | 1,576,668.5 |
| Other costs (start up, permits, etc.) (USD) | 1,006,998.3 | 1,576,668.5 |
| Working capital (USD) | 805,598.6 | 1,261,334.8 |
| Total capital investment (USD) | 16,917,571.2 | 26,488,030.6 |
| Costing Components | FD Process | TFO Process |
|---|---|---|
| Labor cost (USD) | 1,504,296 | 1,504,296 |
| Maintenance cost (USD) | 1,353,866.4 | 1,353,866.4 |
| Labor burden (USD) | 113,616.9 | 214,100 |
| Property insurance (USD) | 1,127,838.1 | 1,765,868.7 |
| Total variable cost (USD) | 14,834,442.6 | 8,791,658.6 |
| Total operating cost (USD) | 18,934,059.9 | 13,629,789.7 |
| Process | MSP (USD/kg) |
|---|---|
| FD process | 1.17 |
| TFO process | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, O.V.; Nie, L.; Alimoradi, H.; Shavandi, A. Waste Apple Pomace Conversion to Acrylic Acid: Economic and Potential Environmental Impact Assessments. Fermentation 2022, 8, 21. https://doi.org/10.3390/fermentation8010021
Okoro OV, Nie L, Alimoradi H, Shavandi A. Waste Apple Pomace Conversion to Acrylic Acid: Economic and Potential Environmental Impact Assessments. Fermentation. 2022; 8(1):21. https://doi.org/10.3390/fermentation8010021
Chicago/Turabian StyleOkoro, Oseweuba Valentine, Lei Nie, Houman Alimoradi, and Amin Shavandi. 2022. "Waste Apple Pomace Conversion to Acrylic Acid: Economic and Potential Environmental Impact Assessments" Fermentation 8, no. 1: 21. https://doi.org/10.3390/fermentation8010021