pH-Based Control Strategies for the Nitrification of High-Ammonium Wastewaters
Abstract
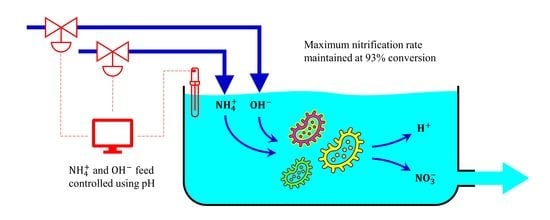
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Apparatus and Instruments
3. Results and Discussion
3.1. Determining the pH Characteristics of Nitrification
3.2. Batch Systems
3.3. Fed-Batch Systems
3.4. Continuous Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Kanter, D.R.; Bartolini, F.; Kugelberg, S.; Leip, A.; Oenema, O.; Uwizeye, A. Nitrogen pollution policy beyond the farm. In Nature Food; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef] [Green Version]
- Castellar, J.A.C.; Formosa, J.; Fernández, A.I.; Jové, P.; Bosch, M.G.; Morató, J.; Arias, C.A. Cork as a sustainable carbon source for nature-based solutions treating hydroponic wastewaters–Preliminary batch studies. Sci. Total. Environ. 2019, 650, 267–276. [Google Scholar] [CrossRef]
- Gagnon, V.; Maltais-Landry, G.; Puigagut, J.; Chazarenc, F.; Brisson, J. Treatment of hydroponics wastewater using constructed wetlands in winter conditions. Water Air Soil Pollut. 2010, 212, 483–490. [Google Scholar] [CrossRef]
- Prystay, W.; Lo, K.V. Treatment of greenhouse wastewater using constructed wetlands. J. Environ. Sci. Health Part. B Pestic. Food Contam. Agric. Wastes 2001, 36, 341–353. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Calvo, M.J.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Exploring nutrient recovery from hydroponics in urban agriculture: An environmental assessment. Resour. Conserv. Recycl. 2020, 155. [Google Scholar] [CrossRef]
- Saxena, P.; Bassi, A. Removal of nutrients from hydroponic greenhouse effluent by alkali precipitation and algae cultivation method. J. Chem. Technol. Biotechnol. 2013, 88, 858–863. [Google Scholar] [CrossRef]
- Chan-Pacheco, C.R.; Valenzuela, E.I.; Cervantes, F.J.; Quijano, G. Novel biotechnologies for nitrogen removal and their coupling with gas emissions abatement in wastewater treatment facilities. In Science of the Total Environment; Elsevier, B.V.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Ronga, D.; Setti, L.; Salvarani, C.; De Leo, R.; Bedin, E.; Pulvirenti, A.; Francia, E. Effects of solid and liquid digestate for hydroponic baby leaf lettuce (Lactuca sativa L.) cultivation. Sci. Hortic. 2019, 244, 172–181. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, K.J.; Asp, H.; Hultberg, M. Utilizing anaerobic digestates as nutrient solutions in hydroponic production systems. Sustainability 2020, 12, 10076. [Google Scholar] [CrossRef]
- Neal, J.; Wilkie, A.C. Anaerobic Digester Effluent as Fertilizer for Hydroponically Grown Tomatoes. J. Undergrad. Res. 2014, 15, 1–5. [Google Scholar]
- Stoknes, K.; Scholwin, F.; Krzesiński, W.; Wojciechowska, E.; Jasińska, A. Efficiency of a novel “Food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Manag. 2016, 56, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Hydroponics Market Size, Share & Trends Analysis Report by Type (Aggregate Systems, Liquid Systems), by Crops (Tomatoes, Lettuce, Peppers, Cucumbers, Herbs), by Region, and Segment Forecasts 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/hydroponics-market (accessed on 17 October 2021).
- Kumar, R.R.; Cho, J.Y. Reuse of hydroponic waste solution. Environ. Sci. Pollut. Res. 2014, 21, 9569–9577. [Google Scholar] [CrossRef]
- Pelayo, L.O.; Hultberg, M.; Bergstrand, K.J.; Larsson-Jönsson, H.; Caspersen, S.; Asp, H. Biogas Digestate in Vegetable Hydroponic Production: pH Dynamics and pH Management by Controlled Nitrification. Waste Biomass Valorization 2021, 12, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Pitts, M.; Stutte, G. Computer model of hydroponics nutrient solution pH control using ammonium. Life Support Biosph. Sci. Int. J. Earth Space 1999, 6, 73–85. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; University of California, College of Agriculture, Agricultural Experiment Station: Berkeley, CA, USA, 1938. [Google Scholar]
- Botheju, D.; Svalheim, O.; Bakke, R. Digestate Nitrification for Nutrient Recovery. Open Waste Manag. J. 2010, 3, 1–12. [Google Scholar] [CrossRef]
- Edgar, M.; Levi, A.; Percy, C.; Gaddafi, L. Analysis of nitrifying microbial community for organic hydroponics. Afr. J. Microbiol. Res. 2018, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.V. Prediction of the optimum pH for ammonia-N oxidation by nitrosomonas Europaea in well-aerated natural and domestic-waste waters. Water Res. 1984, 18, 561–566. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms, 10th ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2003; p. 1019. [Google Scholar]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef] [Green Version]
- Andreottola, G.; Foladori, P.; Ragazzi, M. On-line control of a SBR system for nitrogen removal from industrial wastewater. Water Sci. Technol. 2001, 43, 93–100. [Google Scholar] [CrossRef]
- Hajsardar, M.; Borghei, S.M.; Hassani, A.H.; Takdastan, A. Simultaneous ammonium and nitrate removal by a modified intermittently aerated sequencing batch reactor (SBR) with multiple filling events. Pol. J. Chem. Technol. 2016, 18, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hao, O.J. pH and Oxidation-Reduction Potential Control Strategy for Optimization of Nitrogen Removal in an Alternating Aerobic-Anoxic System All use subject to JSTOR Terms and Conditions pH and Control Nitrogen Strategy Removal Potential of for Optimization in an A. Water Environ. Res. 2001, 73, 95–102. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, D.I.; Keller, J. Effect of temperature and free ammonia on nitrification and nitrite accumulation in landfill leachate and analysis of its nitrifying bacterial community by FISH. Bioresour. Technol. 2006, 97, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Cytryn, E.; Levkovitch, I.; Negreanu, Y.; Dowd, S.; Frenk, S.; Silber, A. Impact of short-term acidification on nitrification and nitrifying bacterial community dynamics in soilless cultivation media. Appl. Environ. Microbiol. 2012, 78, 6576–6582. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, S.; Fatemi, L.; Fallahi, E. Effect of ammonium: Nitrate ratio on yield, calcium concentration, and photosynthesis rate in strawberry. J. Plant. Nutr. 2006, 29, 1273–1285. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rooyen, I.L.; Brink, H.G.; Nicol, W. pH-Based Control Strategies for the Nitrification of High-Ammonium Wastewaters. Fermentation 2021, 7, 319. https://doi.org/10.3390/fermentation7040319
van Rooyen IL, Brink HG, Nicol W. pH-Based Control Strategies for the Nitrification of High-Ammonium Wastewaters. Fermentation. 2021; 7(4):319. https://doi.org/10.3390/fermentation7040319
Chicago/Turabian Stylevan Rooyen, Ignatius Leopoldus, Hendrik Gideon Brink, and Willie Nicol. 2021. "pH-Based Control Strategies for the Nitrification of High-Ammonium Wastewaters" Fermentation 7, no. 4: 319. https://doi.org/10.3390/fermentation7040319