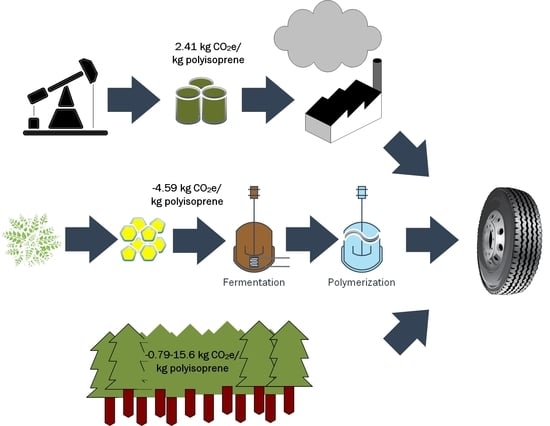
Bio-Based Polyisoprene Can Mitigate Climate Change and Deforestation in Expanding Rubber Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.W.; Na, D.; Park, J.M.; Lee, J.; Choi, S.; Lee, S.Y. Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat. Chem. Biol. 2012, 8, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Machine learning applications in systems metabolic engineering. Curr. Opin. Biotechnol. 2020, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nat. Cell Biol. 2000, 403, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.S.; Cantor, C.R.; Collins, J.J. Construction of a genetic toggle switch in Escherichia coli. Nat. Cell Biol. 2000, 403, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Venetz, J.E.; Del Medico, L.; Wölfle, A.; Schächle, P.; Bucher, Y.; Appert, D.; Tschan, F.; Flores-Tinoco, C.E.; van Kooten, M.; Guennoun, R.; et al. Chemical synthesis rewriting of a bacterial genome to achieve design flexibility and biological functionality. Proc. Natl. Acad. Sci. USA 2019, 116, 8070–8079. [Google Scholar] [CrossRef] [Green Version]
- Heracleous, E.; Pachatouridou, E.; Louie, L.; Dugar, D.; Lappas, A.A. Efficient Route for the Production of Isoprene via Decarboxylation of Bioderived Mevalonolactone. ACS Catal. 2020, 10, 9649–9661. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Qin, B.; Li, Y.; Sun, Y.; Su, S.; Xian, M. Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl. Microbiol. Biotechnol. 2011, 90, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W.; Jin, Y.-S. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol. 2004, 63, 495–509. [Google Scholar] [CrossRef]
- Dharmadi, Y.; Murarka, A.; Gonzalez, R. Anaerobic fermentation of glycerol by Escherichia coli: A new platform for metabolic engineering. Biotechnol. Bioeng. 2006, 94, 821–829. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Ahrends, A.; Hollingsworth, P.M.; Ziegler, A.D.; Fox, J.; Chen, H.; Su, Y.; Xu, J. Current trends of rubber plantation expansion may threaten biodiversity and livelihoods. Glob. Environ. Chang. 2015, 34, 48–58. [Google Scholar] [CrossRef]
- Warren-Thomas, E.M.; Edwards, D.P.; Bebber, D.P.; Chhang, P.; Diment, A.N.; Evans, T.D.; Lambrick, F.; Maxwell, J.F.; Nut, M.; O’Kelly, H.J.; et al. Protecting tropical forests from the rapid expansion of rubber using carbon payments. Nat. Commun. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaupper, T.; Hetz, S.; Kolb, S.; Yoon, S.; Horn, M.A.; Ho, A. Deforestation for oil palm: Impact on microbially mediated methane and nitrous oxide emissions, and soil bacterial communities. Biol. Fertil. Soils 2020, 56, 287–298. [Google Scholar] [CrossRef]
- Morais, A.; Dworakowska, S.; Reis, A.; Gouveia, L.; Matos, C.T.; Bogdał, D.; Lukasik, R. Chemical and biological-based isoprene production: Green metrics. Catal. Today 2015, 239, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Shale Gas Revolution: An Opportunity for the Production of Biobased Chemicals? Angew. Chem. Int. Ed. 2013, 52, 11980–11987. [Google Scholar] [CrossRef]
- Markets and Markets. Isoprene Market by Type (Polymer grade, Chemical grade), Application (Polyisoprene, Styrene Isoprene Styrene, Isobutyl Isoprene Rubber), End-Use Industry (Tires, Non-tires, Adhesives), and Region—Global Forecast to 2021 Title. 2017. Available online: https://www.researchandmarkets.com/reports/4209856/isoprene-market-by-type-polymer-grade-chemical (accessed on 12 September 2021).
- IEA. Global EV Outlook 2020: Entering the Decade of Electric Drive? OECD: Paris, France, 2020; p. 276. [Google Scholar]
- Asghar, U.; Masoom, A.; Javed, A.; Abbas, A. Economic Analysis of Isoprene Production from Good Year Scientific Process. Am. J. Chem. Eng. 2020, 8, 63. [Google Scholar] [CrossRef]
- Luo, Z.; Abdel-Haleem, H. Phenotypic diversity of USDA guayule germplasm collection grown under different irrigation conditions. Ind. Crop. Prod. 2019, 142, 111867. [Google Scholar] [CrossRef]
- Bhadra, S.; Mohan, N.; Parikh, G.; Nair, S. Possibility of artocarpus heterophyllus latex as an alternative source for natural rubber. Polym. Test. 2019, 79, 106066. [Google Scholar] [CrossRef]
- Soratana, K.; Rasutis, D.; Azarabadi, H.; Eranki, P.L.; Landis, A.E. Guayule as an alternative source of natural rubber: A comparative life cycle assessment with Hevea and synthetic rubber. J. Clean. Prod. 2017, 159, 271–280. [Google Scholar] [CrossRef]
- ISO. ISO 14040:2006—Environmental Management—Life Cycle Assessment—Principles and Framework. Available online: https://www.iso.org/standard/37456.html (accessed on 9 October 2020).
- ISO. ISO 14044:2006—Environmental Management—Life Cycle assessment—Requirements and Guidelines. Available online: https://www.iso.org/standard/38498.html (accessed on 9 October 2020).
- Arvidsson, R.; Tillman, A.; Sandén, B.A.; Janssen, M.; Nordelöf, A.; Kushnir, D.; Molander, S. Environmental Assessment of Emerging Technologies: Recommendations for Prospective LCA. J. Ind. Ecol. 2018, 22, 1286–1294. [Google Scholar] [CrossRef] [Green Version]
- Thomassen, G.; Van Dael, M.; Van Passel, S.; You, F. How to assess the potential of emerging green technologies? Towards a prospective environmental and techno-economic assessment framework. Green Chem. 2019, 21, 4868–4886. [Google Scholar] [CrossRef]
- Sorunmu, Y.; Billen, P.; Spatari, S. A review of thermochemical upgrading of pyrolysis bio-oil: Techno-economic analysis, life cycle assessment, and technology readiness. GCB Bioenergy 2020, 12, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Riazi, B.; Karanjikar, M.; Spatari, S. Renewable Rubber and Jet Fuel from Biomass: Evaluation of Greenhouse Gas Emissions and Land Use Trade-offs in Energy and Material Markets. ACS Sustain. Chem. Eng. 2018, 6, 14414–14422. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Jiang, Y.; Sun, B.; Gao, F.; Yang, S. Synergy between methylerythritol phosphate pathway and mevalonate pathway for isoprene production in Escherichia coli. Metab. Eng. 2016, 37, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Pre Consultants, SimaPro 9.1. 2020. Available online: https://simapro.com/wp-content/uploads/2020/10/FullUpdateInstructionsToSimaPro911.pdf (accessed on 21 September 2021).
- Sphera Gabi 9. Life Cycle Assessment LCA Software. 2020. Available online: http://www.gabi-software.com/international/index/ (accessed on 9 October 2020).
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Conversion of Lignocellulosic Biomass to Ethanol; NREL Technical Report; NREL/TP-5100-51400; National Renewable Energy Laboratory: Golden, CO, USA, 2011; Volume 303, pp. 1–176. Available online: http://www.osti.gov/bridge (accessed on 15 March 2021).
- Pourhashem, G.; Adler, P.R.; McAloon, A.J.; Spatari, S. Cost and greenhouse gas emission tradeoffs of alternative uses of lignin for second generation ethanol. Environ. Res. Lett. 2013, 8, 025021. [Google Scholar] [CrossRef] [Green Version]
- Spatari, S.; Bagley, D.; MacLean, H.L. Life cycle evaluation of emerging lignocellulosic ethanol conversion technologies. Bioresour. Technol. 2010, 101, 654–667. [Google Scholar] [CrossRef]
- MacLean, H.L.; Spatari, S. The contribution of enzymes and process chemicals to the life cycle of ethanol. Environ. Res. Lett. 2009, 4. [Google Scholar] [CrossRef]
- Lybarger, H.M. Isoprene. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Honeywell. UniSim Process Design Suite; Honeywell: Calgary, AB, Canada, 2021. [Google Scholar]
- Alnajrani, M.N.; Mair, F.S. Bidentate forms of β-triketimines: Syntheses, characterization and outstanding performance of enamine–diimine cobalt complexes in isoprene polymerization. Dalton Trans. 2016, 45, 10435–10446. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Roovers, J.E.L. Synthesis and solution properties of linear, four-branched, and six-branched star polyisoprenes. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 2521–2533. [Google Scholar] [CrossRef]
- Spatari, S.; Betz, M.; Florin, H.; Baitz, M.; Faltenbacher, M. Using GaBi 3 to perform life cycle assessment and life cycle engineering. Int. J. Life Cycle Assess. 2001, 6, 81–84. [Google Scholar] [CrossRef]
- Ciliberti, C.; Jordaan, S.M.; Smith, S.V.; Spatari, S. A life cycle perspective on land use and project economics of electricity from wind and anaerobic digestion. Energy Policy 2016, 89, 52–63. [Google Scholar] [CrossRef]
- Xu, C.C.; Dessbesell, L.; Zhang, Y.; Yuan, Z. Lignin valorization beyond energy use: Has lignin’s time finally come? Biofuels Bioprod. Biorefin. 2021, 15, 32–36. [Google Scholar] [CrossRef]
- Belaud, J.-P.; Prioux, N.; Vialle, C.; Buche, P.; Destercke, S.; Barakat, A.; Sablayrolles, C. Intensive Data and Knowledge-Driven Approach for Sustainability Analysis: Application to Lignocellulosic Waste Valorization Processes. Waste Biomass Valoriz. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Davis, R.E.; Grundl, N.J.; Tao, L.; Biddy, M.J.; Tan, E.C.; Beckham, G.T.; Humbird, D.; Thompson, D.N.; Roni, M.S. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels and Coproducts: 2018 Biochemical Design Case Update: Biochemical Deconstruction and Conversion of Biomass to Fuels and Products via Integrated Biorefinery Path. 2018; p. 147. Available online: www.nrel.gov/publications (accessed on 21 April 2021).
- Jawjit, W.; Pavasant, P.; Kroeze, C. Evaluating environmental performance of concentrated latex production in Thailand. J. Clean. Prod. 2015, 98, 84–91. [Google Scholar] [CrossRef]
- Jawjit, W.; Kroeze, C.; Rattanapan, S. Greenhouse gas emissions from rubber industry in Thailand. J. Clean. Prod. 2010, 18, 403–411. [Google Scholar] [CrossRef]
- De Blécourt, M.; Brumme, R.; Xu, J.; Corre, M.D.; Veldkamp, E. Soil Carbon Stocks Decrease following Conversion of Secondary Forests to Rubber (Hevea brasiliensis) Plantations. PLoS ONE 2013, 8, e69357. [Google Scholar] [CrossRef] [Green Version]
- Larnaudie, V.; Bule, M.; San, K.-Y.; Vadlani, P.V.; Mosby, J.; Elangovan, S.; Karanjikar, M.; Spatari, S. Life cycle environmental and cost evaluation of renewable diesel production. Fuel 2020, 279, 118429. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Brown, R.C. Life cycle assessment of the production of hydrogen and transportation fuels from corn stover via fast pyrolysis. Environ. Res. Lett. 2013, 8, 025001. [Google Scholar] [CrossRef] [Green Version]
- Cornish, K. Similarities and differences in rubber biochemistry among plant species. Phytochemistry 2001, 57, 1123–1134. [Google Scholar] [CrossRef]





| Parameter | Unit | Amount | Data sources and assumptions |
|---|---|---|---|
| Sugar yield from pre-treatment and enzymatic hydrolysis | g sugars/ g feedstock | 0.597 | National Renewable Energy Laboratory (NREL) [33] |
| Fermentation conversion | g isoprene/ g sugars | 0.267 | Modelled in UniSim using experimental data from Yang et al. [29] |
| Fermenter temperature | °C | 35 | Kirk-Othmer Encyclopedia [37] |
| Heat duty in fermenter | kJ/kg Isoprene | 1708 | Fermenter duty taken from UniSim simulation |
| Condenser duty after fermenter | kJ/kg Isoprene | 87.3 | Condenser duty taken from UniSim simulation |
| Polymerization conversion | % | 99.9 | Experimental data from Alnajrani et al. [39] |
| Polymerization activationenergy | kcal/mol | 3.3 | Hadjichristidis et al. [40] |
| Surplus electricity coproduct | kWh/kg polyisoprene | 2.7 | Reference calculated on assumption of selling electricity coproduct to the grid |
| Input | Quantity | Unit |
|---|---|---|
| Corn stover (CS) feedstock | 5.53 | kg |
| Feedstock yield | 9.7 | ton/ha/year |
| Collection | 1.7 | MJ |
| Nutrient replacement | N 27.7 P 10 K 50.9 | g g g |
| N2O emissions | 305.8 | g |
| Change in soil organic carbon | 1115.6 | g |
| Transportation diesel | 25.7 | mL |
| Enzymes | 50.9 | g |
| Lime | 160.4 | g |
| Diammonium phosphate | 10.5 | g |
| Sulfuric acid | 143.8 | g |
| Electricity required (supplied onsite) | 0.142 | kWh |
| Oxygen from air | 235 | g |
| Output | ||
| Net co-produced electricity | 2.7 | kWh |
| Impact Category | Unit |
|---|---|
| Fine particulate matter | PM2.5 equivalent |
| Stratospheric ozone depletion | kg CFC-11 equivalent |
| Freshwater eutrophication | kg Phosphorous equivalent |
| Marine eutrophication | kg Nitrogen equivalent |
| Freshwater ecotoxicity | kg 1,4-Dichlorobenzene equivalent |
| Marine ecotoxicity | kg 1,4-Dichlorobenzene equivalent |
| Human non-carcinogenic toxicity | kg 1,4-Dichlorobenzene equivalent |
| Terrestrial acidification | kg Sulfur Dioxide equivalent |
| Fossil depletion | kg oil equivalent |
| Pathway | Description 1 |
|---|---|
| DF | Direct fermentation pathway of sugars to isoprene. |
| DF-BC | Direct fermentation pathway of sugars to isoprene including biogenic carbon. |
| FMBE | Indirect fermentation via methyl butenol |
| FMBE-BC | Indirect fermentation via methyl butenol including biogenic carbon |
| NR | Natural rubber |
| NR-BC | Natural rubber including biogenic carbon |
| SR | Synthetic rubber |
| Feedstock Source | Land Use (ha/Metric ton Polyisoprene) | Net GHG Emissions (kg CO2e/kg Polyisoprene) | Net GHG Emissions (kg CO2e/kg Polyisoprene) excl. Biogenic Carbon |
|---|---|---|---|
| DF | 0.25 a to 0.59 a | −4.59 a | 0.79 a |
| FMBE | 0.3 b to 0.7 b | −3.64 a | 2.5 a |
| NR | 1.6 c | −0.79 d to 15.9 b | 1.18 d |
| SR | 0 b | 2.41 e | 2.41 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batten, R.; Karanjikar, M.; Spatari, S. Bio-Based Polyisoprene Can Mitigate Climate Change and Deforestation in Expanding Rubber Production. Fermentation 2021, 7, 204. https://doi.org/10.3390/fermentation7040204
Batten R, Karanjikar M, Spatari S. Bio-Based Polyisoprene Can Mitigate Climate Change and Deforestation in Expanding Rubber Production. Fermentation. 2021; 7(4):204. https://doi.org/10.3390/fermentation7040204
Chicago/Turabian StyleBatten, Rahamim, Mukund Karanjikar, and Sabrina Spatari. 2021. "Bio-Based Polyisoprene Can Mitigate Climate Change and Deforestation in Expanding Rubber Production" Fermentation 7, no. 4: 204. https://doi.org/10.3390/fermentation7040204