Conducting Polymer Metallic Emerald: Magnetic Measurements of Nanocarbons/Polyaniline and Preparation of Plastic Composites
Abstract
:1. Introduction
2. Synthesis
3. Results
3.1. Fourie Transform Infrared
3.2. Ultraviolet-Visible
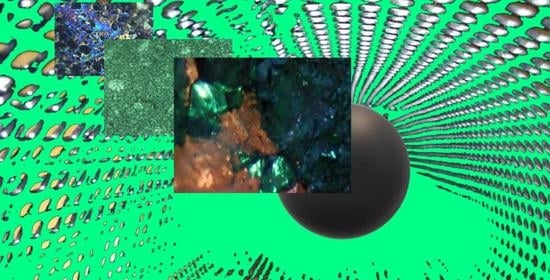
3.3. Surface Image
3.4. Synchrotron X-ray Diffraction
3.5. Electron Spin Resonance (ESR)
3.6. Superconducting Quantum Interference Device Measurements
3.7. I-V Character
4. Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, B.; Monthioux, M.; Luzzi, D. Encapsulated C60 in carbon nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Shinohara, H. Exploring the inner space of carbon nanotubes. Jpn. J. Appl. Phys. 2018, 57, 020101. [Google Scholar] [CrossRef]
- Service, R.F. Solid-State Physics: Nanotube ‘Peapods’ show electrifying promise. Science 2001, 292, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, J. Electronic properties of peapods: Effects of fullerene rotation and different types of tube. J. Phys. Cond. Mat. 2004, 16, 1401–1408. [Google Scholar] [CrossRef]
- Sandeep, G.; Felix, B.; Alicja, B.; Maria, D.; Ronny, S.; Franziska, S.; Jürgen, T.; Thomas, G.; Ewa, B.-P.; Jamie, H.W.; et al. In situ observations of fullerene fusion and ejection in carbon nanotubes. Nanoscale 2010, 2, 2077–2079. [Google Scholar] [CrossRef]
- Kausar, A. Carbon peapod encapsulating fullerene and inorganic nanoparticle toward polymeric nanocomposite: Tailored features and promises. Poly. Plast. Technol. Mater. 2022, 61, 1481–1502. [Google Scholar] [CrossRef]
- Kausar, A. Fullerene nanowhisker nanocomposite—Current stance and high-tech opportunities. Poly. Plast. Technol. Mater. 2022, 61, 593–608. [Google Scholar] [CrossRef]
- Wolfgang, K.M.; Jiménez, P.; Payne, N.O.; Shepherd, R.; Shepherd, R.L.; Castell, P.; Panhuis, M.I.H.; Benito, A.M. Nanofibrilar-Polyaniline/Carbon Nanotube Composites: Aqueous Dispersions and Films. J. Nanosci. Nanotech. 2009, 9, 6157–6163. [Google Scholar] [CrossRef]
- Iwamori, R.; Sato, R.; Kuwabara, J.; Kanbara, T. Nonstoichiometric hydroarylation polyaddition for synthesis of pyrrole-based poly(arylenevinylene)s. Polym. Chem. 2022, 13, 379–382. [Google Scholar] [CrossRef]
- Chen, X.; Ichige, A.; Chen, J.; Fukushima, I.; Kuwabara, J.; Kanbara, T. Facile Access to Conjugated Polymers under Aerobic Conditions via Pd-Catalyzed Direct Arylation and Aryl Amination Polycondensation. Polymer 2020, 207, 122927. [Google Scholar] [CrossRef]
- Tamao, K.; Sumitani, K.; Kumada, M.J. Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes. J. Am. Chem. Soc. 1972, 94, 4374. [Google Scholar] [CrossRef]
- Komaba, K.; Goto, H. Soliton excitations in liquid crystal polyacetylene. Mol. Cryst. Liq. Cryst. 2020, 703, 69. [Google Scholar] [CrossRef]
- Goto, H. Doping-Dedoping-Driven Optic Effect of π-Conjugated Polymers Prepared in Cholesteric-Liquid-Crystal Electrolytes. Phys. Rev. Lett. 2007, 98, 253901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Somasiri, N.L.D.; Epstein, A.J. Polyaniline: Synthesis and Characterization of the Emeraldine Oxidation State by Elemental Analysis. In Conducting Polymers; Alcácer, L., Ed.; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar] [CrossRef]
- Godovsky, D.Y.; Varfolomeev, A.E.; Zaretsky, D.F.; Chandrakanthi, N.R.L.; Kűndig, A.; Wederc, C.; Caseri, W. Preparation of nanocomposites of polyaniline and inorganic semiconductors. J. Mater. Chem. 2001, 11, 2465–2469. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Ghosh, A.; Mondal, A.; Banerjee, D. Polyaniline-single walled carbon nanotube composite—A photocatalyst to degrade rose bengal and methyl orange dyes under visible-light illumination. RSC Adv. 2017, 7, 36403–36415. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, R.; Goto, H. Preparation of polyaniline in organic liquid crystal solvent. Mol. Cryst. Liq. Cryst. 2021, 738, 1–7. [Google Scholar] [CrossRef]
- Goto, H.; Komaba, K.; Yonehara, T.; Miyashita, R.; Kumai, R. Synthesis of polyaniline in organic solvents. Polym.-Plast. Technol. Mater. 2022, 61, 1593–1606. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshikawa, Y.; Miyashita, R.; Yonehara, T.; Komaba, K.; Kumai, R.; Goto, H. Conducting Polymer Metallic Emerald: Magnetic Measurements of Nanocarbons/Polyaniline and Preparation of Plastic Composites. C 2022, 8, 60. https://doi.org/10.3390/c8040060
Koshikawa Y, Miyashita R, Yonehara T, Komaba K, Kumai R, Goto H. Conducting Polymer Metallic Emerald: Magnetic Measurements of Nanocarbons/Polyaniline and Preparation of Plastic Composites. C. 2022; 8(4):60. https://doi.org/10.3390/c8040060
Chicago/Turabian StyleKoshikawa, Yusuke, Ryo Miyashita, Takuya Yonehara, Kyoka Komaba, Reiji Kumai, and Hiromasa Goto. 2022. "Conducting Polymer Metallic Emerald: Magnetic Measurements of Nanocarbons/Polyaniline and Preparation of Plastic Composites" C 8, no. 4: 60. https://doi.org/10.3390/c8040060