Investigation of Electron Transfer Mechanistic Pathways of Ferrocene Derivatives in Droplet at Carbon Electrode
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Voltammetric Parameters and Electrochemical Cells
2.3. Acquisition and Presentation of Voltammetric Data
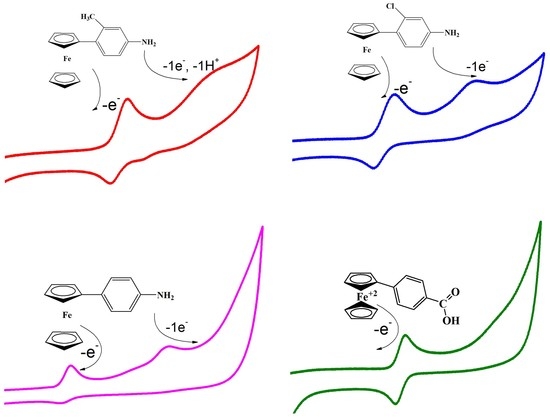
3. Results and Discussion
3.1. Cyclic Voltammetry
3.2. Differential Pulse Voltammetry
3.3. Square Wave Voltammetry
4. Proposed Redox Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, H.; Jørgensen, P.; Helgaker, T. The molecular structure of ferrocene. J. Chem. Phys. 1996, 104, 9528–9530. [Google Scholar] [CrossRef]
- Fabbrizzi, L. The ferrocenium/ferrocene couple: A versatile redox switch. ChemTexts 2020, 6, 22. [Google Scholar] [CrossRef]
- Bhatt, V. Essentials of Coordination Chemistry: A Simplified Approach with 3D Visuals; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Saenger, W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Bersier, P.M.; Bersier, J.; Klingert, B. Electrochemistry of cyclodextrins and cyclodextrin inclusion complexes. Electroanalysis 1991, 3, 443–455. [Google Scholar] [CrossRef]
- Heinze, K.; Lang, H. Ferrocene—Beauty and Function; ACS Publications: Washington, WA, USA, 2013; Volume 32, pp. 5623–5625. [Google Scholar]
- Amer, W.A.; Wang, L.; Amin, A.M.; Ma, L.; Yu, H. Recent progress in the synthesis and applications of some ferrocene derivatives and ferrocene-based polymers. J. Inorg. Organomet. Polym. Mater. 2010, 20, 605–615. [Google Scholar] [CrossRef]
- Corra, S.; Curcio, M.; Baroncini, M.; Silvi, S.; Credi, A. Photoactivated artificial molecular machines that can perform tasks. Adv. Mater. 2020, 32, 1906064. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chen, K.-J.; Wu, T.-H.; Chang, H.-K.; Tsuchido, Y.; Sei, Y.; Chen, P.-L.; Horie, M. Ring rotation of ferrocene in interlocked molecules in single crystals. Chem. Sci. 2021, 12, 3871–3875. [Google Scholar] [CrossRef]
- Kaifer, A.E.; Gómez-Kaifer, M. Supramolecular Electrochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Shim, N.Y.; Bernards, D.A.; Macaya, D.J.; DeFranco, J.A.; Nikolou, M.; Owens, R.M.; Malliaras, G.G. All-plastic electrochemical transistor for glucose sensing using a ferrocene mediator. Sensors 2009, 9, 9896–9902. [Google Scholar] [CrossRef]
- Cunningham, L.; Benson, A.; Guiry, P.J. Recent developments in the synthesis and applications of chiral ferrocene ligands and organocatalysts in asymmetric catalysis. Org. Biomol. Chem. 2020, 18, 9329–9370. [Google Scholar] [CrossRef]
- Altun, A.; Apetrei, R.-M.; Camurlu, P. Reagentless amperometric glucose biosensors: Ferrocene-tethering and copolymerization. J. Electrochem. Soc. 2020, 167, 107507. [Google Scholar] [CrossRef]
- Soon, G.H.; Deasy, M.; Dempsey, E. An Electrochemical Evaluation of Novel Ferrocene Derivatives for Glutamate and Liver Biomarker Biosensing. Biosensors 2021, 11, 254. [Google Scholar] [CrossRef]
- Guven, N.; Apetrei, R.-M.; Camurlu, P. Next step in 2nd generation glucose biosensors: Ferrocene-loaded electrospun nanofibers. Mater. Sci. Eng. C 2021, 128, 112270. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, C.; Du, D.; Xiang, J.; Yao, S.; Hu, E.; Liu, S.; Tong, Y.; Wong, W.Y.; Zhao, Q. Donor–acceptor metallopolymers containing ferrocene for brain inspired memristive devices. Adv. Electron. Mater. 2020, 6, 2000841. [Google Scholar] [CrossRef]
- Salas, M.; Gordillo, B.; González, F.J. Current measurements as a tool to characterise the H-bonding between 1-ferrocenylmethylthymine and 9-octyladenine: A voltammetric and chronoamperometric analysis. J. Electroanal. Chem. 2004, 574, 33–39. [Google Scholar] [CrossRef]
- Camm, K.D.; Furtado, S.J.; Gott, A.L.; McGowan, P.C. Synthesis and structural studies of bis-amino-functionalised ferrocene salts and ferrocenium salts. Polyhedron 2004, 23, 2929–2936. [Google Scholar] [CrossRef]
- Padeste, C.; Steiger, B.; Grubelnik, A.; Tiefenauer, L. Molecular assembly of redox-conductive ferrocene–streptavidin conjugates—towards bio-electrochemical devices. Biosens. Bioelectron. 2004, 20, 545–552. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Wan, Q.; Wang, X.-L.; Sun, Z.-D.; Zhu, Z.-Q.; Xian, Y.-Z.; Jin, L.-T.; Yamamoto, K. Amperometric sensor based on ferrocene-doped silica nanoparticles as an electron transfer mediator for the determination of glucose in rat brain coupled to in vivo microdialysis. J. Electroanal. Chem. 2004, 571, 133–138. [Google Scholar] [CrossRef]
- Asaftei, S.; Walder, L. Covalent layer-by-layer type modification of electrodes using ferrocene derivatives and crosslinkers. Electrochim. Acta 2004, 49, 4679–4685. [Google Scholar] [CrossRef]
- Pandey, P.; Upadhyay, S.; Upadhyay, A. Electrochemical sensors based on functionalized ormosil-modified electrodes—Role of ruthenium and palladium on the electrocatalysis of nadh and ascorbic acid. Sens. Actuators B Chem. 2004, 102, 126–131. [Google Scholar] [CrossRef]
- Pandey, P.; Upadhyay, S.; Shukla, N.; Sharma, S. Studies on the electrochemical performance of glucose biosensor based on ferrocene encapsulated ORMOSIL and glucose oxidase modified graphite paste electrode. Biosens. Bioelectron. 2003, 18, 1257–1268. [Google Scholar] [CrossRef]
- Pandey, P.; Upadhyay, S.; Tiwari, I.; Sharma, S. A Novel Ferrocene-Encapsulated Palladium-Linked Ormosil-Based Electrocatalytic Biosensor. The Role of the Reactive Functional Group. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 1519–1527. [Google Scholar] [CrossRef]
- Gritzner, G.; Kuta, J. Recommendations on reporting electrode potentials in nonaqueous solvents (Recommendations 1983). Pure Appl. Chem. 1984, 56, 461–466. [Google Scholar] [CrossRef]
- Abraham, K. Directions in secondary lithium battery research and development. Electrochim. Acta 1993, 38, 1233–1248. [Google Scholar] [CrossRef]
- Nawaz, H.; Akhter, Z.; Yameen, S.; Siddiqi, H.M.; Mirza, B.; Rifat, A. Synthesis and biological evaluations of some Schiff-base esters of ferrocenyl aniline and simple aniline. J. Organomet. Chem. 2009, 694, 2198–2203. [Google Scholar] [CrossRef]
- Kerman, K.; Mahmoud, K.A.; Kraatz, H.-B. An electrochemical approach for the detection of HIV-1 protease. Chem. Commun. 2007, 37, 3829–3831. [Google Scholar] [CrossRef]
- Sarhan, A.A.; Ibrahim, M.S.; Kamal, M.M.; Mitobe, K.; Izumi, T. Synthesis, cyclic voltammetry, and UV–Vis studies of ferrocene-dithiafulvalenes as anticipated electron-donor materials. Mon. Chem.-Chem. Mon. 2009, 140, 315–323. [Google Scholar] [CrossRef]
- Lal, B.; Kanwal, A.; Altaf, A.A.; Badshah, A.; Asghar, F.; Akhter, S.; Ullah, S.; Khan, S.I.; Tahir, M.N. Synthesis, crystal structure, spectral and electrochemical characterization, DNA binding and free radical scavenging studies of ferrocene-based thioureas. J. Coord. Chem. 2019, 72, 2376–2392. [Google Scholar] [CrossRef]
- Asghar, F.; Munir, S.; Fatima, S.; Murtaza, B.; Patujo, J.; Badshah, A.; Butler, I.S.; Taj, M.B.; Tahir, M.N. Ferrocene-functionalized anilines as potent anticancer and antidiabetic agents: Synthesis, spectroscopic elucidation, and DFT calculations. J. Mol. Struct. 2022, 1249, 131632. [Google Scholar] [CrossRef]
- Asghar, F.; Badshah, A.; Fatima, S.; Zubair, S.; Butler, I.S.; Tahir, M.N. Biologically active meta-substituted ferrocenyl nitro and amino complexes: Synthesis, structural elucidation, and DFT calculations. J. Organomet. Chem. 2017, 843, 48–61. [Google Scholar] [CrossRef]
- Nawaz, S.; Asghar, F.; Patujo, J.; Fatima, S.; Murtaza, B.; Munir, S.; Naz, M.; Badshah, A.; Butler, I.S. New ferrocene-integrated multifunctional guanidine surfactants: Synthesis, spectroscopic elucidation, DNA interaction studies, and DFT calculations. New J. Chem. 2022, 46, 185–198. [Google Scholar] [CrossRef]
- Muller, T.J.; Conradie, J.; Erasmus, E. A spectroscopic, electrochemical and DFT study of para-substituted ferrocene-containing chalcone derivatives: Structure of FcCOCHCH (p-tBuC6H4). Polyhedron 2012, 33, 257–266. [Google Scholar] [CrossRef]
- Cardona, R.A.; Hernández, K.; Pedró, L.E.; Otaño, M.R.; Montes, I.; Guadalupe, A.R. Electrochemical and spectroscopical characterization of ferrocenyl chalcones. J. Electrochem. Soc. 2010, 157, F104. [Google Scholar] [CrossRef]
- Terki, B.; Lanez, T. Anodic behaviour investigation of (ferrocenylmethyl) trimethylammonium cation. Ann. Sci. Technol. 2007, 1, 6. [Google Scholar]
- Kennedy, K.G.; Miles, D.T. Electrochemistry of ferrocene-modified monolayer-protected gold nanoclusters at reduced temperatures. J. Undergrad. Chem. Res. 2004, 4, 145. [Google Scholar]
- Neghmouche, N.; Lanez, T. Electrochemical properties of ferrocene in aqueous and organic mediums at glassy carbon electrode. Recent Trends Phys. Chem. Int. J. 2013, 1, 1–3. [Google Scholar]
- Seiwert, B.; Karst, U. Ferrocene-based derivatization in analytical chemistry. Anal. Bioanal. Chem. 2008, 390, 181–200. [Google Scholar] [CrossRef]
- Pournaghi-Azar, M.; Ojani, R. Catalytic oxidation of ascorbic acid by some ferrocene derivative mediators at the glassy carbon electrode. Application to the voltammetric resolution of ascorbic acid and dopamine in the same sample. Talanta 1995, 42, 1839–1848. [Google Scholar] [CrossRef]
- Shah, A.; Nosheen, E.; Munir, S.; Badshah, A.; Qureshi, R.; Muhammad, N.; Hussain, H. Characterization and DNA binding studies of unexplored imidazolidines by electronic absorption spectroscopy and cyclic voltammetry. J. Photochem. Photobiol. B Biol. 2013, 120, 90–97. [Google Scholar] [CrossRef]
- Nosheen, E.; Shah, A.; Badshah, A.; Hussain, H.; Qureshi, R.; Ali, S.; Siddiq, M.; Khan, A.M. Electrochemical oxidation of hydantoins at glassy carbon electrode. Electrochim. Acta 2012, 80, 108–117. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Shah, A.; Nosheen, E.; Qureshi, R.; Yasinzai, M.M.; Lunsford, S.K.; Dionysiou, D.D.; Siddiq, M.; Badshah, A.; Ali, S. Electrochemical characterization, detoxification and anticancer activity of didodecyldimethylammonium bromide. Int. J. Org. Chem. 2011, 1, 183. [Google Scholar] [CrossRef]
- Munir, S.; Shah, A.; Zafar, F.; Badshah, A.; Wang, X.; Rehman, Z.-U.; Hussain, H.; Lunsford, S.K. Redox behavior of a derivative of vitamin K at a glassy carbon electrode. J. Electrochem. Soc. 2012, 159, G112. [Google Scholar] [CrossRef]
- Munir, S.; Shah, A.; Rauf, A.; Badshah, A.; Hussain, H.; Ahmad, Z. Redox behavior of juglone in buffered aq.: Ethanol media. C. R. Chim. 2013, 16, 1140–1146. [Google Scholar] [CrossRef]
- Munir, S.; Shah, A.; Rauf, A.; Badshah, A.; Lunsford, S.K.; Hussain, H.; Khan, G.S. Redox behavior of a novel menadiol derivative at glassy carbon electrode. Electrochim. Acta 2013, 88, 858–864. [Google Scholar] [CrossRef]
- Golea, D.; Diculescu, V.; Enache, A.; Butu, A.; Tugulea, L.; Brett, A.O. Electrochemical evaluation of dsDNA—Liposomes interactions. Dig. J. Nanomater. Biostruct. 2012, 7, 1333–1342. [Google Scholar]
- Shah, A.; Khan, A.M.; Qureshi, R.; Ansari, F.L.; Nazar, M.F.; Shah, S.S. Redox behavior of anticancer chalcone on a glassy carbon electrode and evaluation of its interaction parameters with DNA. Int. J. Mol. Sci. 2008, 9, 1424–1434. [Google Scholar] [CrossRef]
- Yardim, Y.; Şentürk, Z. Voltammetric behavior of indole-3-acetic acid and kinetin at pencil-lead graphite electrode and their simultaneous determination in the presence of anionic surfactant. Turk. J. Chem. 2011, 35, 413–426. [Google Scholar] [CrossRef]















| Compound | Redox Signal | Voltammetric Parameter | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ipa (µA) | Ep (V) | Ep−Ep1/2 (V) | ∆Ep (V) | Ipa/ Ipc | D/10−7 cm2 s−1 | H+s | Reversibility | ||
| FMA | 1a | 13.2 | 0.31 | - | 0.12 | 4.12 | 9.03 | - | QR |
| 1c | −3.2 | 0.23 | - | ||||||
| 2a | 15 | 0.84 | 0.08 | - | - | 1 | IR | ||
| CFA | 1a | 14.6 | 0.27 | - | 0.13 | 2.92 | 9.18 | - | QR |
| 1c | −5 | 0.11 | - | ||||||
| 2a | 16 | 0.85 | 0.07 | - | - | IR | |||
| FA | 1a | 8.32 | 0.17 | - | 0.07 | 2.97 | 5.53 | - | QR |
| 1c | −2.8 | 0.1 | - | ||||||
| 2a | 18 | 0.85 | 0.07 | IR | |||||
| FBA | 1a | 9.90 | 0.36 | 0.05 | 0.07 | 1.5 | 5.64 | - | QR |
| 1c | 6.22 | 0.27 | 0.06 | ||||||
| Compound | ΔEp (V) | ψ | ksh × 10−4 (cm/sec) | α = (E1/2−Epc)/Epa−Epc |
|---|---|---|---|---|
| FMA | 115 | 0.394 | 8.9 | 0.476 |
| CFA | 150 | 0.212 | 4.8 | 0.453 |
| FA | 105 | 0.496 | 8.7 | 1.1 |
| FBA | 0.073 | 1.51 | 6.7 | 5.69 |
| Compounds | Signal 1a | Signal 2a | ||
|---|---|---|---|---|
| D (cm2/s) | ksh (cm/s) | D (cm2/s) | ksh (cm/s) | |
| FMA | 9.03 × 10−7 | 8.90 × 10−4 | 8.92 × 10−6 | 4.66 × 10−4 |
| CFA | 9.18 × 10−7 | 8.47 × 10−4 | 6.79 × 10−6 | 1.40 × 10−4 |
| FA | 5.53 × 10−7 | 5.45 × 10−4 | 5.752 × 10−6 | 1.30 × 10−4 |
| FBA | 5.64 × 10−7 | 6.74 × 10−4 | - | - |
| Compounds | s | R2 | m | LOD (µA) (1a) | LOD (µA) (2a) | LOQ (µA) (1a) | LOQ (µA) (2a) |
|---|---|---|---|---|---|---|---|
| FMA | 0.432 | 0.97 | 7.10 | 0.183 | 0.112 | 0.61 | 0.028 |
| CFA | 0.269 | 0.97 | 5.72 | 0.141 | 0.113 | 0.54 | 0.094 |
| FA | 0.209 | 0.97 | 3.67 | 0.171 | 0.119 | 0.56 | 0.390 |
| FBA | 0.238 | 0.94 | 4.58 | 0.113 | - | 0.52 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayaz, S.; Shah, A.; Munir, S. Investigation of Electron Transfer Mechanistic Pathways of Ferrocene Derivatives in Droplet at Carbon Electrode. C 2022, 8, 45. https://doi.org/10.3390/c8030045
Ayaz S, Shah A, Munir S. Investigation of Electron Transfer Mechanistic Pathways of Ferrocene Derivatives in Droplet at Carbon Electrode. C. 2022; 8(3):45. https://doi.org/10.3390/c8030045
Chicago/Turabian StyleAyaz, Sidra, Afzal Shah, and Shamsa Munir. 2022. "Investigation of Electron Transfer Mechanistic Pathways of Ferrocene Derivatives in Droplet at Carbon Electrode" C 8, no. 3: 45. https://doi.org/10.3390/c8030045