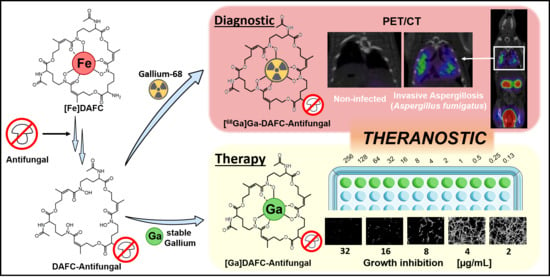
Antifungal Siderophore Conjugates for Theranostic Applications in Invasive Pulmonary Aspergillosis Using Low-Molecular TAFC Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Synthesis of Antifungal Siderophore Conjugates
2.2. Radiolabeling
2.3. In Vitro Experiments
2.3.1. Distribution Coefficient (LogD)
2.3.2. Protein Binding
2.3.3. Serum Stability
2.3.4. Uptake and Competition Assay
2.3.5. Growth Promotion Assay
2.3.6. Antifungal Susceptibility Assays
2.4. Animal Experiments
2.4.1. In Vivo Stability and Ex Vivo Biodistribution
2.4.2. Invasive Pulmonary Aspergillosis Model in Rats
2.4.3. PET/CT Imaging
3. Results
3.1. Synthesis and Radiolabeling
3.2. In Vitro Characterization
3.2.1. LogD, Protein Binding, and Serum Stability
3.2.2. Uptake and Competition Assay
3.2.3. Growth Promotion Assay
3.2.4. Antifungal Susceptibility Assays
3.3. In Vivo Experiments
3.3.1. In Vivo Stability and Biodistribution
3.3.2. PET/CT Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, rv13–rv165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenks, J.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latgé, J.-P. Aspergillus fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [Green Version]
- Moura, S.; Cerqueira, L.; Almeida, A. Invasive pulmonary aspergillosis: Current diagnostic methodologies and a new molecular approach. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1393–1403. [Google Scholar] [CrossRef]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Lucas, J.A.; Arendrup, M.C.; Bowyer, P.; Brinkmann, A.J.F.; Denning, D.W.; Dyer, P.S.; Fisher, M.C.; Geenen, P.L.; Gisi, U.; et al. The one health problem of azole resistance in Aspergillus fumigatus: Current insights and future research agenda. Fungal Biol. Rev. 2020, 34, 202–214. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Iron—A Key Nexus in the Virulence of Aspergillus fumigatus. Front. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N.; Haynes, K.; Haas, H. Siderophore Biosynthesis But Not Reductive Iron Assimilation Is Essential for Aspergillus fumigatus Virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [Green Version]
- Haas, H.; Schoeser, M.; Lesuisse, E.; Ernst, J.F.; Parson, W.; Abt, B.; Winkelmann, G.; Oberegger, H. Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C. Biochem. J. 2003, 371, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Haas, H.; Laverman, P.; Schrettl, M.; Franssen, G.M.; Blatzer, M.; Decristoforo, C. 68Ga-Triacetylfusarinine C and 68Ga-Ferrioxamine E for Aspergillus Infection Imaging: Uptake Specificity in Various Microorganisms. Mol. Imaging Biol. 2014, 16, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Raymond-Bouchard, I.; Carroll, C.S.; Nesbitt, J.R.; Henry, K.A.; Pinto, L.J.; Moinzadeh, M.; Scott, J.K.; Moore, M.M. Structural Requirements for the Activity of the MirB Ferrisiderophore Transporter of Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Franssen, G.M.; Haas, H.; Laverman, P.; Hörtnagl, C.; Schrettl, M.; Helbok, A.; Lass-Flörl, C.; Decristoforo, C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1175–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeopookum, P.; Summer, D.; Pfister, J.; Orasch, T.; Lechner, B.E.; Petrik, M.; Novy, Z.; Matuszczak, B.; Rangger, C.; Haas, H.; et al. Modifying the Siderophore Triacetylfusarinine C for Molecular Imaging of Fungal Infection. Mol. Imaging Biol. 2019, 21, 1097–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, J.; Lichius, A.; Summer, D.; Haas, H.; Kanagasundaram, T.; Kopka, K.; Decristoforo, C. Live-cell imaging with Aspergillus fumigatus-specific fluorescent siderophore conjugates. Sci. Rep. 2020, 10, 15519. [Google Scholar] [CrossRef]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C.; et al. Hybrid Imaging of Aspergillus fumigatus Pulmonary Infection with Fluorescent, 68Ga-Labelled Siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenas, M.E.; Cruz, M.C.; Del Poeta, M.; Chung, N.; Perfect, J.R.; Heitman, J. Antifungal Activities of Antineoplastic Agents:Saccharomyces cerevisiae as a Model System To Study Drug Action. Clin. Microbiol. Rev. 1999, 12, 583–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmina, E. Topical Eflornithine. Am. J. Clin. Dermatol. 2001, 2, 202. [Google Scholar] [CrossRef]
- Levin, V.A.; Ictech, S.E.; Hess, K.R. Clinical importance of eflornithine (α-difluoromethylornithine) for the treatment of malignant gliomas. CNS Oncol. 2018, 7, CNS16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuemmerle, A.; Schmid, C.; Kande, V.; Mutombo, W.; Ilunga, M.; Lumpungu, I.; Mutanda, S.; Nganzobo, P.; Ngolo, D.; Kisala, M.; et al. Prescription of concomitant medications in patients treated with Nifurtimox Eflornithine Combination Therapy (NECT) for T.b. gambiense second stage sleeping sickness in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2020, 14, e0008028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, C.; Brun, R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003, 90, S49–S52. [Google Scholar] [CrossRef]
- Beckmann, N.; Schafferer, L.; Schrettl, M.; Binder, U.; Talasz, H.; Lindner, H.; Haas, H. Characterization of the Link between Ornithine, Arginine, Polyamine and Siderophore Metabolism in Aspergillus fumigatus. PLoS ONE 2013, 8, e67426. [Google Scholar] [CrossRef] [PubMed]
- Boeke, J.D.; La Croute, F.; Fink, G.R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. MGG 1984, 197, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 Years, Two Molecules and (Nearly) No Resistance. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brandhorst, T.T.; Klein, B.S. Uncertainty surrounding the mechanism and safety of the post-harvest fungicide fludioxonil. Food Chem. Toxicol. 2019, 123, 561–565. [Google Scholar] [CrossRef]
- Bigham, M.; Copes, R. Thiomersal in vaccines: Balancing the risk of adverse effects with the risk of vaccine-preventable disease. Drug Saf. 2005, 28, 89–101. [Google Scholar] [CrossRef]
- Clements, C.J. The evidence for the safety of thiomersal in newborn and infant vaccines. Vaccine 2004, 22, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Magos, L.; Clarkson, T.W. Overview of the clinical toxicity of mercury. Ann. Clin. Biochem. 2006, 43, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Zhai, C.; Summer, D.; Rangger, C.; Haas, H.; Haubner, R.; Decristoforo, C. Fusarinine C, a novel siderophore-based bifunctional chelator for radiolabeling with Gallium-68. J. Label. Compd. Radiopharm. 2015, 58, 209–214. [Google Scholar] [CrossRef]
- Pontecorvo, G.; Roper, J.A.; Chemmons, L.M.; Macdonald, K.D.; Bufton, A.W.J. The Genetics of Aspergillus nidulans. Adv. Genet. 1953, 5, 141–238. [Google Scholar] [PubMed]
- Pfister, J.; Bata, R.; Hubmann, I.; Hörmann, A.A.; Gsaller, F.; Haas, H.; Decristoforo, C. Siderophore Scaffold as Carrier for Antifungal Peptides in Therapy of Aspergillus Fumigatus Infections. J. Fungi 2020, 6, 367. [Google Scholar] [CrossRef]
- Ocak, M.; Helbok, A.; Rangger, C.; Peitl, P.K.; Nock, B.A.; Morelli, G.; Eek, A.; Sosabowski, J.K.; Breeman, W.A.P.; Reubi, J.C.; et al. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1426–1435. [Google Scholar] [CrossRef] [Green Version]
- Skriba, A.; Pluhacek, T.; Palyzova, A.; Novy, Z.; Lemr, K.; Hajduch, M.; Petrik, M.; Havlicek, V. Early and Non-invasive Diagnosis of Aspergillosis Revealed by Infection Kinetics Monitored in a Rat Model. Front. Microbiol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, R.W.; Rossato, L.; Valero, C.; Lagrou, K.; Colombo, A.L.; Goldman, G.H. Potential of Gallium as an Antifungal Agent. Front. Cell. Infect. Microbiol. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef] [Green Version]
- Braun, V.; Pramanik, A.; Gwinner, T.; Köberle, M.; Bohn, E. Sideromycins: Tools and antibiotics. BioMetals 2009, 22, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mislin, G.L.A.; Schalk, I.J. Siderophore-dependent iron uptake systems as gates for antibiotic Trojan horse strategies against Pseudomonas aeruginosa. Metallomics 2014, 6, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Gumienna-Kontecka, E.; Carver, P.L. Building a Trojan Horse: Siderophore-Drug Conjugates for the Treatment of Infectious Diseases. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; De Gruyter: Berlin, Germany, 2019; Volume 19, pp. 331–358. [Google Scholar]
- Al Shaer, D.; Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Hydroxamate siderophores: Natural occurrence, chemical synthesis, iron binding affinity and use as Trojan horses against pathogens. Eur. J. Med. Chem. 2020, 208, 112791. [Google Scholar] [CrossRef] [PubMed]
- Dietl, A.-M.; Misslinger, M.; Aguiar, M.M.; Ivashov, V.; Teis, D.; Pfister, J.; Decristoforo, C.; Hermann, M.; Sullivan, S.M.; Smith, L.R.; et al. The Siderophore Transporter Sit1 Determines Susceptibility to the Antifungal VL-2397. Antimicrob. Agents Chemother. 2019, 63, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [Green Version]
- Chitambar, C.R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Ghosh, M.; Niu, C.; Malouin, F.; Moellmann, U.; Miller, M.J. Iron transport-mediated drug delivery using mixed-ligand siderophore- β-lactam conjugates. Chem. Biol. 1996, 3, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.J.; Zhu, H.; Xu, Y.; Wu, C.; Walz, A.J.; Vergne, A.; Roosenberg, J.M.; Moraski, G.; Minnick, A.A.; McKee-Dolence, J.; et al. Utilization of microbial iron assimilation processes for the development of new antibiotics and inspiration for the design of new anticancer agents. BioMetals 2009, 22, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Wencewicz, T.A.; Long, T.E.; Möllmann, U.; Miller, M.J. Trihydroxamate Siderophore–Fluoroquinolone Conjugates Are Selective Sideromycin Antibiotics that Target Staphylococcus aureus. Bioconjug. Chem. 2013, 24, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Neumann, W.; Sassone-Corsi, M.; Raffatellu, M.; Nolan, E.M. Esterase-Catalyzed Siderophore Hydrolysis Activates an Enterobactin–Ciprofloxacin Conjugate and Confers Targeted Antibacterial Activity. J. Am. Chem. Soc. 2018, 140, 5193–5201. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef]
- Petrik, M.; Haas, H.; Dobrozemsky, G.; Lass-Florl, C.; Helbok, A.; Blatzer, M.; Dietrich, H.; Decristoforo, C. 68Ga-Siderophores for PET Imaging of Invasive Pulmonary Aspergillosis: Proof of Principle. J. Nucl. Med. 2010, 51, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Henneberg, S.; Hasenberg, A.; Maurer, A.; Neumann, F.; Bornemann, L.; Gonzalez-Menendez, I.; Kraus, A.; Hasenberg, M.; Thornton, C.R.; Pichler, B.J.; et al. Antibody-guided in vivo imaging of Aspergillus fumigatus lung infections during antifungal azole treatment. Nat. Commun. 2021, 12, 1707. [Google Scholar] [CrossRef]
- Ferreira, K.; Hu, H.Y.; Fetz, V.; Prochnow, H.; Rais, B.; Müller, P.P.; Brönstrup, M. Multivalent Siderophore–DOTAM Conjugates as Theranostics for Imaging and Treatment of Bacterial Infections. Angew. Chem. Int. Ed. 2017, 56, 8272–8276. [Google Scholar] [CrossRef]






| [68Ga]Ga-TAFC * | [68Ga]Ga-DAFC-Eflornithine | [68Ga]Ga-DAFC-Fludioxonil | [68Ga]Ga-DAFC-Thiomersal | [68Ga]Ga-DAFC-FOA | [68Ga]Ga-DAFC-Cy5 ** | ||
|---|---|---|---|---|---|---|---|
| Distributioncoefficient | LogD (pH 7.4) | −2.08 ± 0.02 | −3.45 ± 0.04 | 1.30 ± 0.02 | 0.25 ± 0.06 | −2.66 ± 0.01 | 1.03 ± 0.10 |
| Protein binding [%] | 30 min | 2.54 ± 1.01 | 11.4 ± 4.8 | 14.6 ± 4.3 | 67.4 ± 2.7 | 2.4 ± 0.4 | 13.7 ± 2.9 |
| 60 min | 3.55 ± 0.68 | 8.8 ± 0.7 | 16.8 ± 5.1 | 71.1 ± 0.8 | 2.6 ± 0.7 | 13.1 ± 2.3 | |
| 120 min | 2.96 ± 0.33 | 10.1 ± 1.9 | 15.0 ± 2.6 | 68.2 ± 1.2 | 3.3 ± 1.1 | 13.7 ± 1.8 | |
| Serum stability | 60 min | 99% | 98% | 96% | 72% | 98% | 99% |
| 120 min | 99% | 99% | 95% | 87% | 98% | 97% | |
| 240 min | 99% | 99% | 95% | 80% | 99% | 97% |
| [68Ga]Ga-DAFC-Eflornithine | [68Ga]Ga-DAFC-Fludioxonil | [68Ga]Ga-DAFC-Thiomersal | [68Ga]Ga-DAFC-FOA | [68Ga]Ga-DAFC-Cy5 | |
|---|---|---|---|---|---|
| Blood | 98.0% | 40.6% | 26.7% | >99.0% | >99.0% |
| Urine | 99.0% | 9.8% | 30.5% | >99.0% | 5.2% |
| Organ | [68Ga]Ga-DAFC-Eflornithine | [68Ga]Ga-DAFC-Fludioxonil | [68Ga]Ga-DAFC-Thiomersal | [68Ga]Ga-DAFC-FOA | [68Ga]Ga-DAFC-Cy5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | 90 min | 45 min | 90 min | 45 min | 90 min | 45 min | 90 min | 45 min | 90 min | |
| Blood | 0.93 ± 0.63 | 0.13 ± 0.03 | 0.17 ± 0.06 | 0.14 ± 0.02 | 10.35 ± 3.35 | 3.21 ± 0.94 | 1.30 ± 0.62 | 0.13 ± 0.04 | 4.38 ± 1.02 | 3.37 ± 0.65 |
| Spleen | 0.38 ± 0.05 | 0.22 ± 0.01 | 0.32 ± 0.06 | 0.30 ± 0.05 | 1.82 ± 0.64 | 0.61 ± 0.12 | 0.33 ± 0.08 | 0.12 ± 0.04 | 1.56 ± 0.15 | 5.58 ± 0.94 |
| Pancreas | 0.31 ± 0.07 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.00 | 1.59 ± 0.61 | 0.45 ± 0.09 | 0.28 ± 0.04 | 0.06 ± 0.02 | 0.56 ± 0.07 | 0.46 ± 0.12 |
| Stomach | 0.28 ± 0.06 | 0.09 ± 0.03 | 0.94 ± 1.12 | 0.25 ± 0.22 | 1.77 ± 0.50 | 0.72 ± 0.24 | 0.40 ± 0.07 | 0.13 ± 0.07 | 3.95 ± 3.13 | 1.77 ± 1.61 |
| Intestine | 0.68 ± 0.08 | 0.51 ± 0.05 | 23.08 ± 2.43 | 25.19 ± 1.18 | 19.41 ± 7.26 | 13.63 ± 1.37 | 2.68 ± 0.54 | 2.52 ± 0.40 | 15.82 ± 0.31 | 24.73 ± 0.34 |
| Kidneys | 23.01 ± 2.23 | 19.35 ± 4.55 | 0.22 ± 0.03 | 0.20 ± 0.03 | 7.43 ± 2.24 | 3.67 ± 0.98 | 2.72 ± 0.21 | 1.75 ± 0.21 | 4.60 ± 0.52 | 3.37 ± 0.68 |
| Liver | 0.55 ± 0.03 | 0.38 ± 0.05 | 2.20 ± 0.49 | 0.83 ± 0.23 | 3.67 ± 0.38 | 2.37 ± 0.83 | 0.70 ± 0.34 | 0.60 ± 0.38 | 19.73 ± 2.25 | 22.95 ± 1.57 |
| Heart | 0.28 ± 0.07 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.06 ± 0.02 | 3.03 ± 0.91 | 0.97 ± 0.14 | 0.33 ± 0.09 | 0.07 ± 0.00 | 1.39 ± 0.11 | 1.25 ± 0.25 |
| Lung | 0.89 ± 0.16 | 0.24 ± 0.04 | 0.32 ± 0.26 | 0.18 ± 0.09 | 7.04 ± 2.67 | 2.96 ± 1.75 | 0.66 ± 0.02 | 0.21 ± 0.06 | 2.95 ± 0.20 | 1.95 ± 0.25 |
| Muscle | 0.33 ± 0.23 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 1.25 ± 0.49 | 0.36 ± 0.10 | 0.20 ± 0.02 | 0.05 ± 0.02 | 0.50 ± 0.12 | 0.45 ± 0.11 |
| Femur | 0.21 ± 0.09 | 0.11 ± 0.04 | 0.07 ± 0.02 | 0.09 ± 0.06 | 1.50 ± 0.72 | 0.56 ± 0.22 | 0.45 ± 0.17 | 0.14 ± 0.03 | 0.80 ± 0.43 | 0.85 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfister, J.; Petrik, M.; Bendova, K.; Matuszczak, B.; Binder, U.; Misslinger, M.; Kühbacher, A.; Gsaller, F.; Haas, H.; Decristoforo, C. Antifungal Siderophore Conjugates for Theranostic Applications in Invasive Pulmonary Aspergillosis Using Low-Molecular TAFC Scaffolds. J. Fungi 2021, 7, 558. https://doi.org/10.3390/jof7070558
Pfister J, Petrik M, Bendova K, Matuszczak B, Binder U, Misslinger M, Kühbacher A, Gsaller F, Haas H, Decristoforo C. Antifungal Siderophore Conjugates for Theranostic Applications in Invasive Pulmonary Aspergillosis Using Low-Molecular TAFC Scaffolds. Journal of Fungi. 2021; 7(7):558. https://doi.org/10.3390/jof7070558
Chicago/Turabian StylePfister, Joachim, Milos Petrik, Katerina Bendova, Barbara Matuszczak, Ulrike Binder, Matthias Misslinger, Alexander Kühbacher, Fabio Gsaller, Hubertus Haas, and Clemens Decristoforo. 2021. "Antifungal Siderophore Conjugates for Theranostic Applications in Invasive Pulmonary Aspergillosis Using Low-Molecular TAFC Scaffolds" Journal of Fungi 7, no. 7: 558. https://doi.org/10.3390/jof7070558