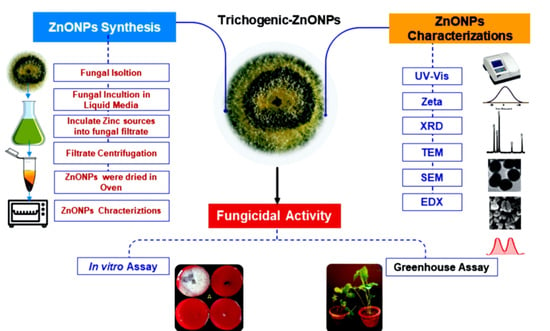
1. Introduction
Cotton (
Gossypium barbadense L.) is a globally important crop that is extensively produced and traded, as well as one of Egypt’s most valuable export crops [
1]. Diseases of cotton seedlings are a worldwide problem caused by pathogenic soil-borne fungi.
Fusarium spp. and
Rhizoctonia solani are among the most pathogenic fungi present in cotton-producing regions in Egypt [
2].
R. solani Kuhn, an anamorph of
Thanatephorus cucumeris (Frank.) Donk [
3], can cause pre-or post-emergence damping-off, seedling blight and root rot in cotton seedlings.
Fusarium spp. are frequently obtained from infected cotton roots and classified as cotton seedling root pathogens [
4].
M. phaseolina (
Tassi) Goid infects over 100 families and 500 plant species all over the world [
5,
6].
M. phaseolina can cause charcoal rot in an abroad range of crops, such as sorghum, soybean, cotton, bean and corn, when conditions are favorable [
7].
Bio-based NP synthesis has received a lot of interest in the last five years. It has eliminated difficult procedures necessary for NPs production utilizing microorganisms, such as fungi, bacteria and yeast, such as microbial cell culture upkeep, prolonged incubation time, several purification steps and so on [
8]. Mycogenic nanoparticles offer advantages, including the formation of a capping from fungal biomolecules, which provides stability and can contribute to various biological activities [
9]. ZnONPs were synthesized from fungal secondary metabolites of three monocultures of
Trichoderma species including,
T. harzianum and
T. reesei. ZnONPs were biogenically produced using a cell filtrate of a strain of
T. harzianum as a reducer and stabilizer agent [
10]. Nevertheless, the microbial synthesis of ZnNPs remains unexplored [
11].
Monoascus purpureus-mediated zinc oxide nanoparticles showed potent antifungal activity against six species of the most common food spoilage fungi [
12].
Sustainable nanomaterials have become a promising option to control plant pathogenic fungi that are responsible for diseases in different crops. Crops treated with safe nano-fungicides will acquire additional value because they are free of chemicals and effective at low doses [
9]. They reduce food and feed spoilage and fungal pathogens and help protect human health and sustain the universal demand for high product quality [
13,
14]. Because of the broad range of uses of zinc oxide nanoparticles (ZnONPs), such as smart UV sensors, they have piqued researchers’ attention [
15], targeted drug delivery [
16], antioxidant activity [
17] biosensors [
18], environmental remediation [
19] and as a drought-tolerant agent as well as nutrient supply of crops [
20]. Moreover, ZnONPs are characterized to be efficient against pathogenic fungi, mostly by their antimicrobial properties according to their photo-oxidizing and photocatalytic effects and considering infection control for the plant host [
21]. Recently detailed reviews introduced the preparation methods and antifungal properties of ZnONPs and their possible antifungal mechanisms for plant diseases management and to improve food quality [
22,
23]. Under in vitro conditions, the biosynthesis of ZnONPs produced from
Trichodermas spp. was used to suppress the development of
Xanthomonas oryzae pv.
oryzae [
10]. An interactive protective impact of ZnONPs on seedling spray/seed soak followed by seedling and biocontrol treatments,
T. harzianum, enhanced plant resistance to
R. solani, the causal organisms of sunflower seedlings damping-off [
24].
Antibacterial, antifungal, antiviral and anti-toxigenic activities against a range of phytopathogens may be achieved using zinc-based nanomaterials, which have targeted antimicrobial capabilities and low to negligible phytotoxic activities (Khan et al., 2021) [
25]. Several applications methods may be used to employ these formulations in open fields or under greenhouse environments [
22,
23,
24]. The use of fungi in the biogenic synthesis of ZnONPs has several benefits, including the production of a capping from fungal biomolecules, which provides stability and can contribute to different biological activities, such as the development of safe nanofungicides. Limited reports have used a
T. harzianum cell filtrate for the production of ZnONPs as only a reducer and stabilizer agent [
26]. Therefore, the main aims of the present study were to 1)synthesis a novel trichogenic-ZnONPs using an easy, eco-friendly, environmentally safe and costless approach, employing fungal metabolites from
T. harzianum strains as a reducing agent and stabilizer to synthesize ZnONPs;2) characterize the synthesized ZnONPs to confirm synthesis, structure, size and NPs morphology; 3) investigate the in vitro and in vivo antifungal activity of mycogenic ZnONPs against soil-borne pathogenic fungi including
R. solani,
Fusarium sp. and
M. phaseolina isolated from infected soil in cotton-growing areas.
4. Discussion
Nanoparticles derived from Trichoderma are still in the early stages of research. Mycogenic ZnONPs utilizing
Trichoderma sp. are more compelling and less harmful to the environment than other methods. Therefore, ZnONPs produced utilizing a cell-free aqueous filtrate of
T. harzianum were shown to have strong antifungal efficacy against the soil-borne pathogen complexes in cotton in this investigation. To confirm whether the synthesized nanoparticles were still stable for one week or changed in the UV results, the absorption spectrum was recorded for the synthesized ZnO sample by Tvivi, T34 and T28 after 2, 4 and 7 days after synthesis. The UV-visible spectrum showed the absorbance peak at 300 nm corresponding to the characteristic band of zinc oxide nanoparticles for all screened Trichoderma isolates at all tested periods. The obtained UV-vis spectrophotometer results were in agreement with Dobrucka et al. [
33], who reported that the maximum absorption of about 310 nm, which is a characteristic band of pure ZnO, verified the presence of ZnONPs biologically with the use of the extract of
Chelidonium majus. Furthermore, there was no additional peak in the spectrum, confirming that the produced products were pure ZnO [
34,
35]. In addition, Perveen et al. [
36] reported that UV-visible spectroscopy investigation showed a peak at 300 nm, which corresponded to the wavelength of ZnO quantum dots’ surface plasmon resonance. The UV-vis spectra of ZnONPs synthesized by
A. niger show that at 390 nm, ZnONPs have a high absorption spectra [
37]. Jamdagni et al. [
38] found that the UV spectrum range of ZnONPs is 320–390 nm, which is a similar result.
The XRD diffraction peaks were 31.84°, 34.52°, 36.33°, 47.63°, 56.71°, 62.96°, 68.13°, 69.18°, 70.16°, 73.21° and 78.56°, which agreed with Sadatzadeh et al., Yedurkar et al. and Malaikozhundanet al. [
39,
40,
41]. The peaks showed the characteristic hexagonal wurtzite structure of ZnO (JCPDS card no. 36–1451) [
42]. The Wurtzite structure was prevalent because it is stable in ambient conditions. It also revealed that the synthesized nanopowder was impurity-free because it lacked any XRD peaks other than zinc oxide peaks. The XRD diffraction peaks matched well with Wurtzite ZnO of the Joint Committee on Powder Diffraction Standards (JCPDS) Card number 36–1451 and were in good accord with the reported literature [
43].
The magnitude of the Zeta potential (−30 mV to +30 mV) indicates the potential stability of the colloidal system [
44,
45,
46]. The Zeta potential is related to the nanoparticles’ stability in the solution. The larger zeta potential values represent a lower degree of aggregation that leads to a higher degree of stability of nanoparticles and a smaller z-averaged hydrodynamic diameter. At lower zeta values, the nanoparticles flocculate early and the stability of the nano-suspension reduces [
44]. The Zeta potential of ZnONPsin the present study was –24.0 mV, which provided evidence that the fabricated nanoparticles were moderately stable, which led to the monodispersity of the particles. The result was in agreement with Divya et al. [
45], who showed a zeta potential of −5.36 mV. Furthermore, Zakharova [
46] reported a zeta potential of 9 mV of ZnONPs had high antimicrobial efficacy and increased ZnONPs toxicity. It has been proven that some nanoparticles have a tendency to aggregate and that this process of aggregation reduces the surface area of nanoparticles. To solve this problem, ZnONPs require extensive ultrasonication in the water bath for a minimum of 15 min.
The results were in agreement with González et al. [
47], who reported that TEM analysis of the synthesized ZnONPs showed spherical, hexagonal and rod shapes. Pillai et al. [
48] reported that the synthesized ZnONPs from an aqueous extract of
Beta vulgaris were spherical with a size of nearly 20 ± 2 nm. Morphology of bio nanoparticles produced from
Cinnamomum tamala was rod-shaped, the particles size within the range 30 ± 3 nm.
TEM results of biosynthesized ZnONPs by
Anacardium occidentale leaf extract confirmed the hexagonal structure with an average particle size of 33 nm [
49]. Our results are in harmony with Ruddaraju et al. [
50]. The results were in agreement with Ruddaraju et al. and Javed et al. [
50,
51]. The SEM images described surface topological details of different nano-objects based on the electron density of the surface due to their higher resolution and bigger field depth [
52]. The agglomeration of ZnONPs might be attributed either due to its polarity and electrostatic attraction between ZnONPs or due to the high surface energy of ZnONPs. The high surface energy of ZnONPs could be originated from an aqueous synthetic medium [
53,
54,
55]. TEM is used to magnify an image by using electromagnetic lenses to magnify an electron beam that travels through thin specimens in a nearly parallel manner. The objective lens is the principal electromagnetic lens. For example, an SEM picture generally shows bigger agglomerated particles, but TEM images have a greater resolution. This means that TEM is superior to SEM in terms of its ability to measure the nanoparticles’ size and has a greater resolution than SEM [
54]. EDX analysis is a chemical microanalysis technique that is used in conjunction with SEM to evaluate elemental composition by detecting X-rays released from the sample during electron beam bombardment [
55]. EDX analysis was in good agreement with XRD results. The EDX results of the present study were in agreement with several reports [
36,
39,
40]. The FTIR spectrum revealed 3398, 3233, 2912, 1640, 1629, 1561, 1461, 1018, 576 and 533 cm
−1 in the current study. The peak at 1640 corresponded to C=O stretching of the functional group. The peak in the range 1556 corresponded to C=C/amine—NH stretching of the aromatic compound [
55].
The wide peak at 3233 cm
−1 may be attributed to an alkenyl group’s C-H stretch, whereas 2104 cm
−1 was moved to –C≡C– stretching vibrations [
40]. Secondary metabolites found in
C. roseus have been linked to the conversion of zinc acetate dihydrate to zinc oxide nanoparticles. The FTIR spectrum showed peaks at 3233, 2104, 1640, 1556, 1399, 1086, 926, 773, 849, 715, 1035, 482, 410 cm
−1 [
56]. Due to stretching alkenyl groups formed by zinc acetate salts and their reduction in ZnONPs, the FTIR spectra peak showed high-intensity broadband of 3233 cm
−1 [
37,
56]. According to the results of our FTIR analysis,
Trichoderma-mediated ZnONPs were synthesized using two distinct processes: reduction and capping. On the surfaces of both the biosynthesized ZnONPs that function as reducing and stabilizing agents, FTIR examination indicated the presence of proteins, amino acids, polyphenols, carboxyl and hydroxyl groups. ZnONPs are characterized by their strong aromatic ring and carboxylic acid appearance in the FTIR bands. According to the results of the FTIR analysis described various mycochemicals such as phenolic, proteins, amino acids, aldehydes, ketone and other functional groups were involved in the reduction, capping and stabilization of zinc oxide NPs (
Figure 8).
In in vitro assay, in addition to inhibiting the vegetative mycelial growth of phytopathogenic fungi, zinc-mediated nanoparticles or composites can kill spores or inhibit spore germination (sporostatic/sporicidal activities) at low concentrations, such as a significant decrease in fungal growth of
B. cinerea and
P. expansum shown on ZnONPs (3 mM/L concentration) treatment [
57]. Yehia and Ahmed [
58] reported the antifungal efficiency of ZnONPs investigated against
F. oxysporum. The maximum inhibition of mycelial growth was seen at (12 mg/L) when
F. oxysporum growth was inhibited by 77 percent. HPLC quantification was used to study the influence of ZnONPs on the mycotoxin fusaric acid. The amount of fusaric acid was lowered from 39.0 to 0.20 mg/g. Scanning electron microscopy showed evident deformation in mycelia that had been treated with ZnONPs in
F. oxysporum, which may cause growth inhibition.
In the present work, in vitro assay, zero fungal growth was investigated with concentrations starting from 20 μg/mL of ZnONPs. Fungicidal properties against three pathogenic fungi were explored in our study. Due to the current dearth of understanding of different aspects of fungal disease biology, these antifungal properties are currently restricted. Lahuf et al. [
24] found that a concentration of 15 mg/mL led to complete inhibition (100%) of
R. solani; however, lower doses of ZnONPs (10, 5 and 2.5 mg/mL) resulted in lower levels of inhibition of
R. solani, by 83.21, 71.03 and 57 percent inhibition, respectively. Furthermore, it was discovered that ZnONPs have fungistatic rather than deadly fungicidal effects on
R. solani. The ZnONPs fungicidal properties revealed that they were diffusible via the growing media [
59]. Shen et al. and Raghupathi et al. [
60,
61] documented the antifungal effects of ZnONPs on microbial populations. It was suggested that zero-valent metal nanoparticles might successfully permeate pathogenic microorganism cell membranes through the lipid bilayer because of their reduced hydrophobicity due to the absence of surface charge [
62]. ZnO showed obvious destruction of the cell walls and plasmolysis of the internal organs of the tested fungi [
63]. In vitro studies against
F. oxysporum,
R. solani and
Sclerotium rolfsii revealed that a mixture of
Trichoderma asperellum and chitosan nanoparticles was better than
Trichoderma alone and carbendazim 0.1% in suppressing pathogen mycelial growth [
64].
In the current study, under greenhouse conditions, the results of the disease management studies of zinc oxide NPs, at two different concentrations (100 and 200 μg/mL), seed treatments for efficacy in the control of damping-off in cotton, compared to Maxim XL and Moncut chemical fungicides, indicated that ZnONPs (200 μg/mL), gave the maximum efficiency in disease control, compared to other treatments in Giza90 for all growth parameters (survival, plant height and dry weight). However, in the case of Giza94 cultivars, ZnONPs (200 μg/mL) NPs were not the best treatment in disease control in the case of survival only. However, it increased the survival significantly compared to infested control, but it was the best treatment in the case of plant height and dry weight. These results indicated that ZnONPs behavior was affected by the cultivar and it may need to be used at different optimum concentrations according to cotton cultivars to give the maximum survival during further future studies. The ZnONPs may form an antifungal layer around cotton seeds that protects cotton seedlings from the three pathogenic fungi. When ZnONPswas used as an antifungal agent against
R. solani at concentrations of 30, 60 and 90 g ml
−1, the second and the third concentration raised the percentages of Giza90 seedlings that survived to 85 and 86%, respectively, compared to 43.5 percent persisted seedlings at the concentration of 30 g m
−1 [
65]. González-Merino et al. [
66] evaluated the antifungal activity of ZnONPs against
F.oxysporum on tomato plants under greenhouse conditions. ZnONPs from 1500 to 3000 g/mL achieved the best plant height with a range of 166.0 to 175.40 cm, a severity of 0.40–0.80 and a disease incidence of 20–40%. In a pot experiment, foliar spraying of ZnONPs was more successful than seed priming in enhancing plant dry weight and controlling the
Pectobacterium betavasculorum,
Meloidogyne incognita and
R. solani, causal disease complex of beetroot
(Beta Vulgaris L.) [
67]. Nevertheless, most ZnONPs may have accumulated on the seed’s exterior surface, with only a few particles moving into the stele and available for biodistribution and bioaccumulation, making seed priming less effective than foliar spray [
68]. ZnONPs are believed to interact with pathogens through mechanical enfolding, which could be one of the main mechanisms of ZnONPs toxicity against
R. solani [
69].