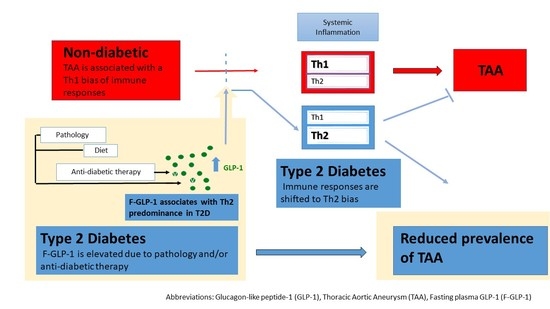
Elevated Glucagon-like Peptide-1 and a Th2 Shift May Support Reduced Prevalence of Thoracic Aortic Aneurysm in Patients with Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. F-GLP-1 Levels
2.3. Cytokines and High-Sensitivity C-Reactive Protein (hsCRP)
2.4. Matrix Metalloproteinase-2 (MMP-2) Activity
2.5. In Vitro GLP-1 Secretion Studies
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. T2D Is Associated with an Increase in IL-6/TNF-α Ratio
3.3. Increased F-GLP-1 Is Associated with a Th2 Inflammatory Profile in T2D Patients
3.4. Th2 Cytokines Are Downregulated in Patients with TAA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isselbacher, E.M.; Cardenas, C.L.L.; Lindsay, M.E. Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation 2016, 133, 2516–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, S.K.; Pedroza, C.; Khalil, Y.A.; Milewicz, D.M. Diabetes and reduced risk for thoracic aortic aneurysms and dissections: A nationwide case-control study. JAHA 2012, 1, e000323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landenhed, M.; Engstrom, G.; Gottsater, A.; Caulfield, M.P.; Hedblad, B.; Newton-Cheh, C.; Melander, O.; Smith, J.G. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: A prospective cohort study. J. Am. Heart Assoc. 2015, 4, e001513. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.L.; Lin, C.L.; Wu, Y.Y.; Shieh, D.C.; Sung, F.C.; Kao, C.H. Advanced complicated diabetes mellitus is associated with a reduced risk of thoracic and abdominal aortic aneurysm rupture: A population-based cohort study. Diabetes/Metab. Res. Rev. 2015, 31, 190–197. [Google Scholar] [CrossRef]
- Avdic, T.; Franzen, S.; Zarrouk, M.; Acosta, S.; Nilsson, P.; Gottsater, A.; Svensson, A.M.; Gudbjornsdottir, S.; Eliasson, B. Reduced Long-Term Risk of Aortic Aneurysm and Aortic Dissection Among Individuals With Type 2 Diabetes Mellitus: A Nationwide Observational Study. J. Am. Heart Assoc. 2018, 7, e007618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffort, J.; Chinetti, G.; Lareyre, F. Glucagon-Like peptide-1: A new therapeutic target to treat abdominal aortic aneurysm? Biochimie 2018, 152, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.R. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol. Behav. 2012, 106, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Krizhanovskii, C.; Ntika, S.; Olsson, C.; Eriksson, P.; Franco-Cereceda, A. Elevated circulating fasting glucagon-like peptide-1 in surgical patients with aortic valve disease and diabetes. Diabetol. Metab. Syndr. 2017, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Pannacciulli, N.; Bunt, J.C.; Koska, J.; Bogardus, C.; Krakoff, J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am. J. Clin. Nutr. 2006, 84, 556–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, V.C.; Ramgolam, V.S.; Bender, J.R. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005, 78, 805–818. [Google Scholar] [CrossRef] [Green Version]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharm. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oviedo-Orta, E.; Bermudez-Fajardo, A.; Karanam, S.; Benbow, U.; Newby, A.C. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology 2008, 124, 42–50. [Google Scholar] [CrossRef]
- Gabrielsson, S.; Soderlund, A.; Nilsson, C.; Lilja, G.; Nordlund, M.; Troye-Blomberg, M. Influence of atopic heredity on IL-4-, IL-12- and IFN-gamma-producing cells in in vitro activated cord blood mononuclear cells. Clin. Exp. Immunol. 2001, 126, 390–396. [Google Scholar] [CrossRef]
- Krizhanovskii, C.; Franco-Cereceda, A. Diabetes, Incretin Therapy and Thoracic Aortic Aneurysm—What Does the Evidence Show? Curr. Vasc. Pharm. 2019, 17, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Muscelli, E.; Mari, A.; Casolaro, A.; Camastra, S.; Seghieri, G.; Gastaldelli, A.; Holst, J.J.; Ferrannini, E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008, 57, 1340–1348. [Google Scholar] [CrossRef] [Green Version]
- Hattori, A.; Kawamura, I.; Yamada, Y.; Kanamori, H.; Aoyama, T.; Ushikoshi, H.; Kawasaki, M.; Nishigaki, K.; Tamemura, G.; Minatoguchi, S. Elevated plasma GLP-1 levels and enhanced expression of cardiac GLP-1 receptors as markers of left ventricular systolic dysfunction: A cross-sectional study. BMJ Open 2013, 3, e003201. [Google Scholar] [CrossRef]
- Lewandowski, K.C.; Banach, E.; Bienkiewicz, M.; Lewinski, A. Matrix metalloproteinases in type 2 diabetes and non-diabetic controls: Effects of short-term and chronic hyperglycaemia. Arch. Med. Sci. 2011, 7, 294–303. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Wang, M.; Sun, Z.; Wang, X.; Miao, J.; Zheng, Z. The predictive value of TNF-alpha and IL-6 and the incidence of macrovascular complications in patients with type 2 diabetes. Acta Diabetol. 2012, 49, 3–7. [Google Scholar] [CrossRef]
- Batra, R.; Suh, M.K.; Carson, J.S.; Dale, M.A.; Meisinger, T.M.; Fitzgerald, M.; Opperman, P.J.; Luo, J.; Pipinos, I.I.; Xiong, W.; et al. IL-1beta (Interleukin-1beta) and TNF-alpha (Tumor Necrosis Factor-alpha) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. Arter. Thromb. Vasc. Biol. 2018, 38, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, J.; Surcel, H.M.; Satta, J.; Teppo, A.M.; Bloigu, A.; Syrjala, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847. [Google Scholar] [CrossRef]
- Treska, V.; Topolcan, O.; Pecen, L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin. Chem. Lab. Med. 2000, 38, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Yakimov, A.O.; Teesdale, M.A.; Coady, M.A.; Dardik, A.; Elefteriades, J.A.; Tellides, G. Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. FASEB J. 2005, 19, 1528–1530. [Google Scholar] [CrossRef]
- Vasu, S.; Moffett, R.C.; McClenaghan, N.H.; Flatt, P.R. Responses of GLP1-secreting L-cells to cytotoxicity resemble pancreatic beta-cells but not alpha-cells. J. Mol. Endocrinol. 2015, 54, 91–104. [Google Scholar] [CrossRef]
- Hadjiyanni, I.; Siminovitch, K.A.; Danska, J.S.; Drucker, D.J. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 2010, 53, 730–740. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; Rizzo, M.R.; Barbieri, M.; Paolisso, P.; D’Onofrio, N.; Giovane, A.; Siniscalchi, M.; Minicucci, F.; Sardu, C.; D’Andrea, D.; et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: Effects of incretin treatment. Diabetes 2015, 64, 1395–1406. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.O.; Kim, H.S.; Youn, J.C.; Shin, E.C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine levels in the serum of healthy subjects. Mediat. Inflamm. 2013, 434010. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Nishanian, P.; Mitsuyasu, R.; Detels, R.; Fahey, J.L. Variables that affect assays for plasma cytokines and soluble activation markers. Clin. Diagn. Lab. Immunol. 1999, 6, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancotto, A.; Wank, A.; Perl, S.; Cook, W.; Olnes, M.J.; Dagur, P.K.; Fuchs, J.C.; Langweiler, M.; Wang, E.; McCoy, J.P. Baseline levels and temporal stability of 27 multiplexed serum cytokine concentrations in healthy subjects. PLoS ONE 2013, 8, e76091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, F.; Williams, A.; Johnson, P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J. Immunol. Methods 2009, 340, 55–64. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntika, S.; Jois, H.; Lång, K.; Olsson, C.; Franco-Cereceda, A.; Björck, H.M.; Krizhanovskii, C. Elevated Glucagon-like Peptide-1 and a Th2 Shift May Support Reduced Prevalence of Thoracic Aortic Aneurysm in Patients with Diabetes. J. Cardiovasc. Dev. Dis. 2021, 8, 143. https://doi.org/10.3390/jcdd8110143
Ntika S, Jois H, Lång K, Olsson C, Franco-Cereceda A, Björck HM, Krizhanovskii C. Elevated Glucagon-like Peptide-1 and a Th2 Shift May Support Reduced Prevalence of Thoracic Aortic Aneurysm in Patients with Diabetes. Journal of Cardiovascular Development and Disease. 2021; 8(11):143. https://doi.org/10.3390/jcdd8110143
Chicago/Turabian StyleNtika, Stelia, Harshitha Jois, Karin Lång, Christian Olsson, Anders Franco-Cereceda, Hanna M. Björck, and Camilla Krizhanovskii. 2021. "Elevated Glucagon-like Peptide-1 and a Th2 Shift May Support Reduced Prevalence of Thoracic Aortic Aneurysm in Patients with Diabetes" Journal of Cardiovascular Development and Disease 8, no. 11: 143. https://doi.org/10.3390/jcdd8110143