Early and Mid-Term Outcomes of Transcatheter Aortic Valve Implantation versus Surgical Aortic Valve Replacement: Updated Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
3. Results
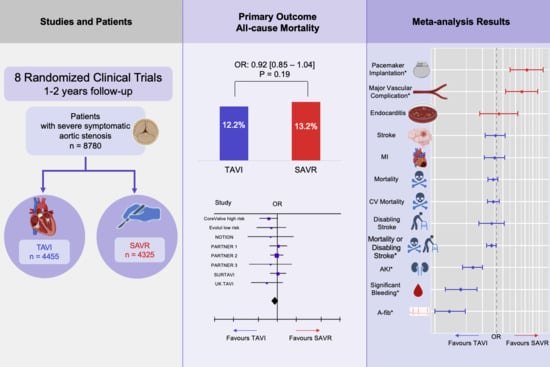
3.1. All-Cause Mortality
3.2. Secondary Outcomes
3.3. Sensitivity Analysis
3.4. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. New Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. New Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. New Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. New Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
- Reardon, M.J.; van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. New Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. New Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter Aortic Valve Implantation vs. Surgical Aortic Valve Replacement for Treatment of Symptomatic Severe Aortic Stenosis: An Updated Meta-Analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. New Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Witberg, G.; Lador, A.; Yahav, D.; Kornowski, R. Transcatheter versus Surgical Aortic Valve Replacement in Patients at Low Surgical Risk: A Meta-Analysis of Randomized Trials and Propensity Score Matched Observational Studies. Catheter. Cardiovasc. Interv. 2018, 92, 408–416. [Google Scholar] [CrossRef]
- Ahmad, Y.; Howard, J.P.; Arnold, A.D.; Madhavan, M.V.; Cook, C.M.; Alu, M.; Mack, M.J.; Reardon, M.J.; Thourani, V.H.; Kapadia, S.; et al. Transcatheter versus Surgical Aortic Valve Replacement in Lower-Risk and Higher-Risk Patients: A Meta-Analysis of Randomized Trials. Eur. Heart. J. 2023, 44, 836–852. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Cochrane: London, UK, 2019. [Google Scholar]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. New Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Fairbairn, T.; Kemp, I.; Young, A.; Ronayne, C.; Barton, J.; Crowe, J.; McQuade, L.; Clarkson, N.; Sionas, M.; Tobin, A.; et al. Effect of Transcatheter Aortic Valve Implantation vs Surgical Aortic Valve Replacement on All-Cause Mortality in Patients with Aortic Stenosis. JAMA 2022, 327, 1875. [Google Scholar] [CrossRef]
- Reardon, M.J.; Adams, D.H.; Kleiman, N.S.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Lee, J.S.; Hermiller, J.B.; Chetcuti, S.; et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2015, 66, 113–121. [Google Scholar] [CrossRef]
- van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, E.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. Self-Expanding Transcatheter vs Surgical Aortic Valve Replacement in Intermediate-Risk Patients: 5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-Year Outcomes for Patients with Aortic Valve Stenosis at Low Surgical Risk Randomized to Transcatheter vs. Surgical Aortic Valve Replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, L.; Ihlemann, N.; Capodanno, D.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Chang, Y.; Steinbrüchel, D.A.; Olsen, P.S.; Petronio, A.S.; et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J. Am. Coll. Cardiol. 2019, 73, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, L.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Ngo, A.T.; Olsen, N.T.; Chang, Y.; Franzen, O.W.; et al. Two-Year Outcomes in Patients with Severe Aortic Valve Stenosis Randomized to Transcatheter Versus Surgical Aortic Valve Replacement: The All-Comers Nordic Aortic Valve Intervention Randomized Clinical Trial. Circ. Cardiovasc. Interv. 2016, 9, e003665. [Google Scholar] [CrossRef]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Rovin, J.D.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Sorajja, P.; Heiser, J.C.; et al. 2-Year Outcomes after Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J. Am. Coll. Cardiol. 2022, 79, 882–896. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jorgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrom, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes from the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-Year Outcomes of Transcatheter Aortic Valve Replacement or Surgical Aortic Valve Replacement for High Surgical Risk Patients with Aortic Stenosis (PARTNER 1): A Randomised Controlled Trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. New Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Webb, J.G.; Nietlispach, F.; Dumont, E.; de Larochelliere, R.; Doyle, D.; Masson, J.-B.; Gutierrez, M.J.; Clavel, M.-A.; Bertrand, O.F.; et al. Acute Kidney Injury Following Transcatheter Aortic Valve Implantation: Predictive Factors, Prognostic Value, and Comparison with Surgical Aortic Valve Replacement. Eur. Heart J. 2010, 31, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Thongprayoon, C.; Cheungpasitporn, W.; Gillaspie, E.A.; Greason, K.L.; Kashani, K.B. Association of Blood Transfusion with Acute Kidney Injury after Transcatheter Aortic Valve Replacement: A Meta-Analysis. World J. Nephrol. 2016, 5, 482. [Google Scholar] [CrossRef]
- Witberg, G.; Steinmetz, T.; Landes, U.; Pistiner Hanit, R.; Green, H.; Goldman, S.; Vaknin-Assa, H.; Codner, P.; Perl, L.; Rozen-Zvi, B.; et al. Change in Kidney Function and 2-Year Mortality After Transcatheter Aortic Valve Replacement. JAMA Netw. Open. 2021, 4, e213296. [Google Scholar] [CrossRef] [PubMed]
- Lerman, T.T.; Levi, A.; Kornowski, R. Meta-Analysis of Short- and Long-Term Clinical Outcomes of the Self-Expanding Evolut R/pro Valve versus the Balloon-Expandable Sapien 3 Valve for Transcatheter Aortic Valve Implantation. Int. J. Cardiol. 2023, 371, 100–108. [Google Scholar] [CrossRef] [PubMed]





| Study | Year | Sample Size | Longest Follow Up | TAVI Valve | STS (mean ± S.D) | EuroScore (mean ± S.D) | Age (mean ± S.D) | Male | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| PARTNER 1 | 2011 | TAVI 348 | 5 years | Sapien (balloon-expandable) | 11.8 ± 3.3 | 29.3 ± 16.5 * | 83.6 ± 6.8 | 58% | Low |
| SAVR 351 | 11.7 ± 3.5 | 29.2 ± 15.6 * | 84.5 ± 6.4 | 57% | |||||
| PARTNER 2 | 2016 | TAVI 1011 | 5 years | Sapien XT (balloon-expandable) | 5.8 ± 2.1 | - | 81.5 ± 6.7 | 54% | Low |
| SAVR 1021 | 5.8 ± 1.9 | - | 81.7 ± 6.7 | 55% | |||||
| PARTNER 3 | 2019 | TAVI 496 | 2 years | Sapien 3 (balloon-expandable) | 1.9 ± 0.7 | 1.5 ± 1.2 | 73.3 ± 5.8 | 68% | Low |
| SAVR 454 | 1.9 ± 0.6 | 1.5 ± 0.9 | 73.6 ± 6.1 | 71% | |||||
| CoreValve | 2014 | TAVI 394 | 5 years | CoreValve (self-exapnding) | 7.3 ± 3.0 | 17.6 ± 13.0 * | 83.2 ± 7.1 | 54% | Some concerns |
| SAVR 401 | 7.5 ± 3.2 | 18.4 ± 12.8 * | 83.5 ± 6.3 | 47% | |||||
| NOTION | 2015 | TAVI 145 | 8 years | CoreValve (self-exapnding) | 2.9 ± 1.6 | 1.9 ± 1.2 | 79.2 ± 4.9 | 54% | Low |
| SAVR 135 | 3.1 ±1.7 | 2.0 ± 1.3 | 79.0 ± 4.7 | 53% | |||||
| SURTAVI | 2017 | TAVI 879 | 5 years | CoreValve/Evolut R (self-exapnding) | 4.4 ± 1.5 | 11.9 ± 7.6 * | 79.9 ± 6.2 | 58% | Low |
| SAVR 867 | 4.5 ± 1.6 | 11.6 ± 8.0 * | 79.8 ± 6.0 | 56% | |||||
| Evolut | 2019 | TAVI 734 | 2 years | CoreValve/Evolut R/Pro (self-exapnding) | 1.9 ± 0.7 | - | 74.0 ± 5.9 | 64% | Low |
| SAVR 734 | 1.9 ± 0.7 | - | 73.8 ± 6.0 | 66% | |||||
| UK TAVI | 2022 | TAVI 458 | 1 year | Various models (12 types) | 2.6 | 2 | 81 | 54% | Low |
| SAVR 455 | 2.7 | 2 | 81 | 53% |
| TAVI | SAVR | OR [95% CI] | |
|---|---|---|---|
| All-cause mortality | 542/4455 (12.2%) | 571/4325 (13.2%) | 0.92 [0.80, 1.04] |
| CV mortality | 341/4459 (7.6%) | 361/4375 (8.3%) | 0.92 [0.79, 1.08] |
| Stroke | 292/4458 (6.6%) | 298/4326 (6.9%) | 0.97 [0.74, 1.27] |
| Disabling stroke | 148/4313 (3.4%) | 167/4191 (4%) | 0.88 [0.62, 1.24] |
| All-cause mortality or disabling stroke | 569/4313 (13.2%) | 624/4191 (14.9%) | 0.87 [0.77, 0.99] |
| MI | 103/4458 (2.3%) | 105/3426 (3.1%) | 0.95 [0.72, 1.25] |
| PPI | 551/3229 (17.1%) | 279/3110 (9%) | 2.28 [1.45, 3.57] |
| MVC | 185/2976 (6.2%) | 106/2869 (3.7%) | 1.99 [1.29, 3.07] |
| Endocarditis | 31/3476 (0.9%) | 28/3458 (0.8%) | 1.07 [0.64, 1.79] |
| Significant bleeding | 454/3434 (13.2%) | 974/3324 (29.3%) | 0.38 [0.25, 0.59] |
| AKI | 90/2942 (3.1%) | 161/2920 (5.5%) | 0.53 [0.40, 0.69] |
| Atrial Fibrillation | 399/3255 (12.3%) | 1032/3116 (33.1%) | 0.28 [0.19, 0.43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerman, T.T.; Levi, A.; Talmor-Barkan, Y.; Kornowski, R. Early and Mid-Term Outcomes of Transcatheter Aortic Valve Implantation versus Surgical Aortic Valve Replacement: Updated Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 157. https://doi.org/10.3390/jcdd10040157
Lerman TT, Levi A, Talmor-Barkan Y, Kornowski R. Early and Mid-Term Outcomes of Transcatheter Aortic Valve Implantation versus Surgical Aortic Valve Replacement: Updated Systematic Review and Meta-Analysis. Journal of Cardiovascular Development and Disease. 2023; 10(4):157. https://doi.org/10.3390/jcdd10040157
Chicago/Turabian StyleLerman, Tsahi T., Amos Levi, Yeela Talmor-Barkan, and Ran Kornowski. 2023. "Early and Mid-Term Outcomes of Transcatheter Aortic Valve Implantation versus Surgical Aortic Valve Replacement: Updated Systematic Review and Meta-Analysis" Journal of Cardiovascular Development and Disease 10, no. 4: 157. https://doi.org/10.3390/jcdd10040157