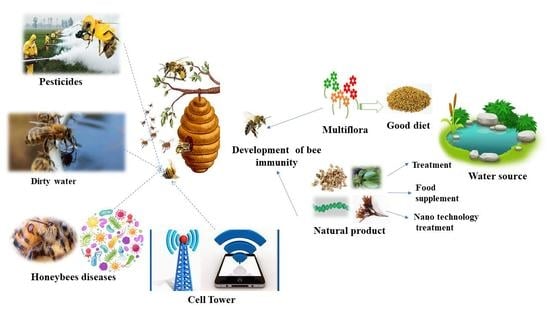
Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health
Abstract
:1. Introduction
2. Honeybee Immunity
3. Main Causes of Honeybee Colony Losses
3.1. Varroa Mite
3.2. Nosema spp.
3.3. Viral Pathogens
3.4. Pesticides
3.5. Malnutrition
3.6. Other Causes
4. Interaction between Different Stressors Affects the Bees Immunocompetence
4.1. Interaction between Pesticides and Pathogens
4.2. Interaction between Pesticides and Poor Nutrition
4.3. Interaction between Pathogens and Poor Nutrition
4.4. Interaction between Parasites and Pathogens
| First Stressor | Second Stressor | Model | Results | References |
|---|---|---|---|---|
| Varroa destructor | Neonicotinoid insecticides | Honeybees | Reduction in survival of long-lived winter honeybees | [115] |
| Deformed wing virus (DWV) | White-eye honeybees pupae | Higher virus replication | [116] | |
| Poor nutrition | Freshly emerged honeybee workers | Reduction in body weight Reduction in abdominal protein level Increased head to abdomen protein ratio | [117] | |
| Nosema ceranae | Insecticide fipronil | Emerging honeybees | Decreased survival | [118] |
| DWV | Newly emerged honeybee workers | Higher virus replication in infected bees in a dose- and nutrition-dependent manner | [119] | |
| Neurotoxic insecticides | Emerging honeybees | Increased mortality of immunity-related genes Strong alteration of midgut immunity | [104] | |
| Thiacloprid | Freshly emerged honeybee workers | Higher Nosema replication | [105] | |
| Microsporidia Nosema | Neonicotinoid (imidacloprid) | Young Africanized honeybees | Higher mortality rate Higher pathogen load | [120] |
| Thiamethoxam | N. ceranae | Larvae and adult honeybees | Gene expression patterns change with time in each treatment | [121] |
| Chronic bee paralysis virus (CBPV) | Nine-day-old bees | Higher viral titers Increased mortality | [122] | |
| CBPV | Emerging honeybees | High pesticides increased mortality without an increase in viral titers Low pesticide has no effect on mortality, however, increases in viral titers | [123] | |
| Imidacloprid | N. ceranae | Honeybee queens | Decreased metabolic and detoxification functions Increased mortality Reduced lifespan | [103] |
| N. ceranae | Honeybees | Higher Nosema infection load Increased mortality of honey bee colonies | [101] | |
| Thiacloprid | Microsporidian N. ceranae and BQCV | Larval and adult honeybees | Elevation of viral load Increased individual mortality | [124] |
| Clothianidin | Nosema spp. | Emerging honeybees | No any synergistic effect | [125] |
| Fipronil and Thiacloprid | N. ceranae | Emerging honeybees | Higher Nosema infection Higher mortality | [126] |
| Poor nutrition | Israeli acute paralysis virus (IAPV). | Honeybees | Elevation of bee mortality | [113] |
| First Stressor | Second Stressor | Model | Results | References |
|---|---|---|---|---|
| Varroa destructor | Clothianidin | Honeybees | Reduced weight and number Up-regulated differentially expressed genes (DEGs) associated with metabolism | [127] |
| Deformed wing virus (DWV) | Africanized honeybees | Inhibition of immunity Reduction in lifespan | [128] | |
| Neonicotinoid insecticide imidacloprid | Honeybees | Reduce homing success of foragers High V. destructor load | [129] | |
| Poor nutrition | Freshly emerged honeybee workers | Death of bee about 40% Ageing enhancement | [117] | |
| Viruses (Acute-Kashmir-Israeli and DWV, acaricide) | Honeybees | Higher virus replication immune-suppression | [130] | |
| DWV | Honeybees | Down-regulation of a member of the NF-Κβ Increase in bees mortality | [41] | |
| Nosema | Herbicide glyphosate and the fungicide difenoconazole | Emerging honeybees | Induces strong physiological disturbances Reduced honeybees longevity | [131] |
| Nosema apis | Neonicotinoid pesticide Thiamethoxam | Honeybee workers | Increase in mortality Reduction in immunocompetence | [132] |
| V. destructor, Nosema spp. | Imidacloprid | Honeybees | Reduced the flight performance by ~24% No effect on colony size | [133] |
| Acaricides | N. ceranae | Newly emerged honeybees | Reduction in ethyl oleate as primer pheromone | [134] |
| Parasites | Pesticides | Honeybees | Increased mortality | [135] |
| Imidacloprid | CBPV | Adult honeybee workers | Higher virus load and mortality in case of higher doses Lower dosages had no influence on virus titer | [106] |
| Clothianidin | Varroa | Honeybees | No elevation of immune gene expression | [74] |
| DWV | Honeybees | Decreased immunocompetence expression Higher replication of the DWV | [102] | |
| Pathogens (RNA viruses, DNA virus, Nosema SP, and beneficial bacteria) | Bumblebees | No increase in viral titers Impairs reproduction of queens and males | [136] | |
| Pathogens | Honeybees | No adverse effect on honey bee colonies No increase in pathogens titers | [137] | |
| Infection with parasites and diseases | Honeybees | No increase in titers of several viruses No impact on success of colony or honey yield | [138] | |
| RoundupVR | Nosema microsporidia | Emerged and adult honeybees | Reduced survival rate and increased food consumption of the bees | [139] |
| Thiamethoxam | N. ceranae | Apis mellifera carnica | Epithelium degeneration Higher mortality | [140] |
| N. ceranae | Larvae and adult honeybees | Highest mortality rate Decreased immunocompetence expression | [121] | |
| DWV | Newly emerged bees | The chance of not returning to the hive after the first flight was raised Decreased survival | [141] | |
| Chronic bee paralysis virus (CBPV) | Emerging honeybees | High pesticides increased mortality without an increase in viral titers Low pesticide has no effect on mortality but increases in viral titers | [123] | |
| CBPV | Adult honeybee workers | Higher viral titers Increased mortality | [122] | |
| Neonicotinoid insecticides | V. destructor | Newly emerged bees | Decreased immunocompetence expression | [142] |
| Insecticide Flupyradifurone (FPF, Sivanto®) | Nutritional stress | Foraging honeybees | Reduced bee survival and food consumption | [110] |
| Pesticides (fipronil, thiamethoxam and boscalid) | N. ceranae | Newly emerged honeybees | Gut microbiota dysbiosis | [143] |
| Neonicotinoid pesticides | Nutritional stress | Honeybees | Reduction in bee survival Reduction in food consumption | [107] |
| Pesticides (dimethoate, clothianidin and fluvalinate) | American foulbrood (AFB) | Honeybees | Higher mortality Reduction in hemocyte counts | [144] |
| Poor nutrition | Virus infection | Honeybees | Elevation of bee mortality | [113] |
| Thiamethoxam | Bumblebees micro-colonies | Slower growth Reduced reproductive efforts | [145] | |
| Thiamethoxam | Apis mellifera ligustica | Negative impact on hypopharyngeal gland development | [146] |
5. Strategies to Enhance Honeybee Immunity
5.1. Fortified Nutrients
5.2. Natural Products as Alternative Sources
5.3. Nanomaterials as Novel Alternative Approaches
5.4. Organizations and Initiatives Directed to Saving the Bees
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691–17700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; Alajmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of apoidea as main pollinators. Ecological and economic impact and implications for human nutrition. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef]
- Goodrich, B.K. Do more bees imply higher fees? Honey bee colony strength as a determinant of almond pollination fees. Food Policy 2019, 83, 150–160. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony collapse disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Baer, B. Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Sci. Rep. 2019, 9, 19753–19762. [Google Scholar] [CrossRef] [Green Version]
- Al Naggar, Y.; Paxton, R.J. Mode of transmission determines the virulence of black queen cell virus in adult honey bees, posing a future threat to bees and apiculture. Viruses 2020, 12, 535. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Paxton, R.J. The novel insecticides flupyradifurone and sulfoxaflor do not act synergistically with viral pathogens in reducing honey bee (Apis mellifera) survival but sulfoxaflor modulates host immunocompetence. Microb. Biotechnol. 2021, 14, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.; Laurent, M.; Consortium, E.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullapudi, E.; Přidal, A.; Pálková, L.; de Miranda, J.R.; Plevka, P. Virion structure of Israeli acute bee paralysis virus. J. Virol. 2016, 90, 8150–8159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Nazzi, F.; Annoscia, D.; Caprio, E.; Di Prisco, G.; Pennacchio, F. Honeybee immunity and colony losses. Entomologia 2014, 2, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Fallon, J.P.; Troy, N.; Kavanagh, K. Pre-exposure of Galleria mellonella larvae to different doses of Aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence 2011, 2, 413–421. [Google Scholar] [CrossRef] [Green Version]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Karlikow, M.; Goic, B.; Saleh, M.-C. RNAi and antiviral defense in Drosophila: Setting up a systemic immune response. Dev. Comp. Immunol. 2014, 42, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Brutscher, L.M.; Flenniken, M.L. RNAi and antiviral defense in the honey bee. J. Immunol. Res. 2015, 2015, 941897. [Google Scholar] [CrossRef] [Green Version]
- Vung, N.N.; Choi, Y.S.; Kim, I. High resistance to Sacbrood virus disease in Apis cerana (Hymenoptera: Apidae) colonies selected for superior brood viability and hygienic behavior. Apidologie 2020, 51, 61–74. [Google Scholar] [CrossRef]
- Goblirsch, M.; Warner, J.F.; Sommerfeldt, B.A.; Spivak, M. Social fever or general immune response? Revisiting an example of social immunity in honey bees. Insects 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.; Bordoni, A.; Cappa, F.; Petrocelli, I.; Pitzalis, M.; Iovinella, I.; Dani, F.R.; Turillazzi, S.; Cervo, R. Increased immunocompetence and network centrality of allogroomer workers suggest a link between individual and social immunity in honeybees. Sci. Rep. 2020, 10, 8928–8939. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evol. Int. J. Org. Evol. 2009, 63, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Spivak, M. Propolis envelope in Apis mellifera colonies supports honey bees against the pathogen, Paenibacillus larvae. Sci. Rep. 2017, 7, 11429–11440. [Google Scholar] [CrossRef] [Green Version]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- Alkhatib, A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef]
- Roger, N.; Michez, D.; Wattiez, R.; Sheridan, C.; Vanderplanck, M. Diet effects on bumblebee health. J. Insect Physiol. 2017, 96, 128–133. [Google Scholar] [CrossRef]
- Aronstein, K.A.; Saldivar, E.; Vega, R.; Westmiller, S.; Douglas, A.E. How Varroa parasitism affects the immunological and nutritional status of the honey bee, Apis mellifera. Insects 2012, 3, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef]
- Gebremedhn, H.; Amssalu, B.; De Smet, L.; De Graaf, D.C. Factors restraining the population growth of Varroa destructor in Ethiopian honey bees (Apis mellifera simensis). PLoS ONE 2019, 14, e0223236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- De Figueiró Santos, J.; Coelho, F.C.; Bliman, P.A. Behavioral modulation of infestation by varroa destructor in bee colonies. Implications for colony stability. PLoS ONE 2016, 11, e0160465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Cabrera, J.; Bumann, H.; Rodríguez-Vargas, S.; Kennedy, P.J.; Krieger, K.; Altreuther, G.; Hertel, A.; Hertlein, G.; Nauen, R.; Williamson, M.S. A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J. Pest Sci. 2018, 91, 1137–1144. [Google Scholar] [CrossRef]
- Morawetz, L.; Köglberger, H.; Griesbacher, A.; Derakhshifar, I.; Crailsheim, K.; Brodschneider, R.; Moosbeckhofer, R. Health status of honey bee colonies (Apis mellifera) and disease-related risk factors for colony losses in Austria. PLoS ONE 2019, 14, e0219293. [Google Scholar] [CrossRef]
- Richards, E.H.; Jones, B.; Bowman, A. Salivary secretions from the honeybee mite, Varroa destructor: Effects on insect haemocytes and preliminary biochemical characterization. Parasitology 2011, 138, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Guzman-Novoa, E. Effect of Varroa destructor, counding and Varroa homogenate on gene expression in brood and adult honey bees. PLoS ONE 2017, 12, e0169669. [Google Scholar] [CrossRef] [Green Version]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielmanowicz, M.G.; Inberg, A.; Lerner, I.M.; Golani, Y.; Brown, N.; Turner, C.L.; Hayes, G.J.R.; Ballam, J.M. Prospective large-scale field study generates predictive model identifying major contributors to colony losses. PLoS Pathog. 2015, 11, e1004816. [Google Scholar] [CrossRef] [Green Version]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Schüler, V.; Horchler, L.L.; Groth, D.; Genersch, E. Long-term temporal trends of Nosema spp. infection prevalence in Northeast Germany: Continuous spread of Nosema ceranae, an emerging pathogen of honey bees (Apis mellifera), but no general replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017, 7, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Grassl, J.; Carson, A.; Simmons, L.W.; Baer, B. Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite Nosema apis. Sci. Rep. 2016, 6, 36649–36659. [Google Scholar] [CrossRef] [Green Version]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef]
- Li, W.; Evans, J.D.; Li, J.; Su, S.; Hamilton, M.; Chen, Y. Spore load and immune response of honey bees naturally infected by Nosema ceranae. Parasitol. Res. 2017, 116, 3265–3274. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Flenniken, M.L. Bee viruses: Ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef]
- Škubnik, K.; Nováček, J.; Füzik, T.; Přidal, A.; Paxton, R.J.; Plevka, P. Structure of deformed wing virus, a major honey bee pathogen. Proc. Natl. Acad. Sci. USA 2017, 114, 3210–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, S.; Brasesco, C.; Negri, P.; Marin, M.; Pagnuco, I.; Szawarski, N.; Reynaldi, F.; Larsen, A.; Eguaras, M.; Maggi, M. Up-regulated pathways in response to Deformed Wing Virus infection in Apis mellifera (Hymenoptera: Apidae). Rev. Soc. Entomol. Argent. 2019, 78, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Arévalo, S.; Vicente-Rubiano, M.; Puerta, F.; Molero, F.; Sánchez-Vizcaíno, J.M. Immune related genes as markers for monitoring health status of honey bee colonies. BMC Vet. Res. 2019, 15, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Di, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [Green Version]
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The iflaviruses sacbrood virus and deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ 2016, 4, e1591. [Google Scholar] [CrossRef] [Green Version]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, e0152685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; DeGrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of Imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera. Insect Sci. 2017, 24, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huang, S.; Heerman, M.; Rodr, C.; Banmeke, O.; Brister, J.R.; Hatcher, E.L.; Cao, L.; Hamilton, M.; Chen, Y. The phylogeny and pathogenesis of sacbrood virus (SBV) infection in european honey bees, Apis mellifera. Viruses 2019, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Liuhao, W.; Jun, G.; Yujie, T.; Yanping, C.; Jie, W.; Jilian, L. Chinese Sacbrood virus infection in Asian honey bees (Apis cerana cerana) and host immune responses to the virus infection. J. Invertebr. Pathol. 2017, 150, 63–69. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Evans, J.D.; Robinson, G.E.; Berenbaum, M.R. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2009, 106, 14790–14795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.; Rivkin, H.; Slabezki, Y.; Chejanovsky, N. Dynamics of the presence of israeli acute paralysis virus in honey bee colonies with colony collapse disorder. Viruses 2014, 6, 2012–2027. [Google Scholar] [CrossRef] [Green Version]
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; VanEngelsdorp, D.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeus, I.; de Miranda, J.R.; de Graaf, D.C.; Wäckers, F.; Smagghe, G. Effect of oral infection with Kashmir bee virus and Israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. J. Invertebr. Pathol. 2014, 121, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, L.J.; Reynaldi, F.J.; Ramello, P.J.; Garcia, M.L.G.; Sguazza, G.H.; Abrahamovich, A.H.; Lucia, M. Detection of honey bee viruses in Argentinian stingless bees (Hymenoptera: Apidae). Insectes Soc. 2018, 65, 191–197. [Google Scholar] [CrossRef]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; AHerniou, E.; Alaux, C.; Conte, Y. Le Viruses in the invasive hornet Vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.N.; Shepherd, T.F.; Rangel, J. The detection of honey bee (Apis mellifera)-associated viruses in ants. Sci. Rep. 2020, 10, 2923–2930. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog 2014, 10, e1004261. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [Green Version]
- Cresswell, J.E.; Thompson, H.M. Comment on “a common pesticide decreases foraging success and survival in honey bees”. Science 2012, 337, 1453. [Google Scholar] [CrossRef] [Green Version]
- Tesovnik, T.; Cizelj, I.; Zorc, M.; Čitar, M.; Božič, J.; Glavan, G.; Narat, M. Immune related gene expression in worker honey bee (Apis mellifera carnica) pupae exposed to neonicotinoid thiamethoxam and Varroa mites (Varroa destructor). PLoS ONE 2017, 12, e0187079. [Google Scholar] [CrossRef] [PubMed]
- Tesovnik, T.; Zorc, M.; Gregorc, A.; Rinehart, T.; Adamczyk, J.; Narat, M. Immune gene expression in developing honey bees (Apis mellifera L.) simultaneously exposed to imidacloprid and Varroa destructor in laboratory conditions. J. Apic. Res. 2019, 58, 730–739. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Osterman, J.; Wintermantel, D.; Locke, B.; Jonsson, O.; Semberg, E.; Onorati, P.; Forsgren, E.; Rosenkranz, P.; Rahbek-Pedersen, T.; Bommarco, R. Clothianidin seed-treatment has no detectable negative impact on honeybee colonies and their pathogens. Nat. Commun. 2019, 10, 692–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siviter, H.; Bailes, E.J.; Martin, C.D.; Oliver, T.R.; Koricheva, J.; Leadbeater, E.; Brown, M.J.F. Agrochemicals interact synergistically to increase bee mortality. Nature 2021, 596, 389–392. [Google Scholar] [CrossRef]
- Alaux, C.; Kemper, N.; Kretzschmar, A.; Le Conte, Y. Brain, physiological and behavioral modulation induced by immune stimulation in honeybees (Apis mellifera): A potential mediator of social immunity? Brain Behav. Immun. 2012, 26, 1057–1060. [Google Scholar] [CrossRef] [Green Version]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Cotter, S.C.; Simpson, S.J.; Raubenheimer, D.; Wilson, K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 2011, 25, 186–198. [Google Scholar] [CrossRef]
- Frizzera, D.; Del Fabbro, S.; Ortis, G.; Zanni, V.; Bortolomeazzi, R.; Nazzi, F.; Annoscia, D. Possible side effects of sugar supplementary nutrition on honey bee health. Apidologie 2020, 51, 594–608. [Google Scholar] [CrossRef]
- Desneux, N.; Bernal, J.S. Genetically modified crops deserve greater ecotoxicological scrutiny. Ecotoxicology 2010, 19, 1642–1644. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Glare, T.R.; Burgess, E.P.J.; Malone, L.A. Effects of plants genetically modified for insect resistance on nontarget organisms. Annu. Rev. Entomol. 2005, 50, 271–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelton, A.M.; Zhao, J.-Z.; Roush, R.T. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 2002, 47, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Qaim, M.; Kouser, S. Genetically modified crops and food security. PLoS ONE 2013, 8, e64879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.J.; Marvier, M.; Huesing, J.; Dively, G.; Huang, Z.Y. A meta-analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLoS ONE 2008, 3, e1415. [Google Scholar] [CrossRef]
- Devillers, J.; Pham-Delègue, M.-H. Using proteins to assess the poteinal impacts of genentically modifiedplants on honey bees. In Honey Bees: Estimating the Environmental Impact of Chemicals; CRC Press: Boca Raton, FL, USA, 2002; pp. 290–304. ISBN 0203218655. [Google Scholar]
- Aldgini, H.M.M.; Al-Abbadi, A.A.; Abu-Nameh, E.S.M.; Alghazeer, R.O. Determination of metals as bio indicators in some selected bee pollen samples from Jordan. Saudi J. Biol. Sci. 2019, 26, 1418–1422. [Google Scholar] [CrossRef]
- Nikolić, T.V.; Kojić, D.; Orčić, S.; Batinić, D.; Vukašinović, E.; Blagojević, D.P.; Purać, J. The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 2016, 164, 98–105. [Google Scholar] [CrossRef]
- Polykretis, P.; Delfino, G.; Petrocelli, I.; Cervo, R.; Tanteri, G.; Montori, G.; Perito, B.; Branca, J.J.V.; Morucci, G.; Gulisano, M. Evidence of immunocompetence reduction induced by cadmium exposure in honey bees (Apis mellifera). Environ. Pollut. 2016, 218, 826–834. [Google Scholar] [CrossRef]
- Rothman, J.A.; Leger, L.; Kirkwood, J.S.; McFrederick, Q.S. Cadmium and selenate exposure affects the honey bee microbiome and metabolome, and bee-associated bacteria show potential for bioaccumulation. Appl. Environ. Microbiol. 2019, 85, e01411-19. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, M.; Gogolewska, H.; Pawelec, N.; Sentkowska, A.; Krasnodębska-Ostręga, B. Comparison of the contents of selected elements and pesticides in honey bees with regard to their habitat. Environ. Sci. Pollut. Res. 2019, 26, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Fisogni, A.; Hautekèete, N.; Piquot, Y.; Brun, M.; Vanappelghem, C.; Michez, D.; Massol, F. Urbanization drives an early spring for plants but not for pollinators. Oikos 2020, 129, 1681–1691. [Google Scholar] [CrossRef]
- Appler, R.H.; Frank, S.D.; Tarpy, D.R. Within-colony variation in the immunocompetency of managed and feral honey bees (Apis mellifera L.) in different urban landscapes. Insects 2015, 6, 912–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabour, K.; Al Naggar, Y.; Masry, S.; Naiem, E.; Giesy, J.P. Cellular alterations in midgut cells of honey bee workers (Apis millefera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci. Total Environ. 2019, 651, 1356–1367. [Google Scholar] [CrossRef]
- AL Naggar, Y.; Dabour, K.; Masry, S.; Sadek, A.; Naiem, E.; Giesy, J.P. Sublethal effects of chronic exposure to CdO or PbO nanoparticles or their binary mixture on the honey bee (Apis millefera L.). Environ. Sci. Pollut. Res. 2020, 27, 19004–19015. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.P.; Kumar, N.R. Changes in honeybee behaviour and biology under the influence of cellphone radiations. Curr. Sci. 2010, 98, 1376–1378. [Google Scholar]
- Santhosh Kumar, S. Colony collapse disorder (CCD) in honey bees caused by EMF radiation. Bioinformation 2018, 14, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Lupi, D.; Tremolada, P.; Colombo, M.; Giacchini, R.; Benocci, R.; Parenti, P.; Parolini, M.; Zambon, G.; Vighi, M. Effects of pesticides and electromagnetic fields on honeybees: A field study using biomarkers. Int. J. Environ. Res. 2020, 14, 107–122. [Google Scholar] [CrossRef]
- Odemer, R.; Odemer, F. Effects of radiofrequency electromagnetic radiation (RF-EMF) on honey bee queen development and mating success. Sci. Total Environ. 2019, 661, 553–562. [Google Scholar] [CrossRef]
- Clermont, A.; Eickermann, M.; Kraus, F.; Hoffmann, L.; Beyer, M. Correlations between land covers and honey bee colony losses in a country with industrialized and rural regions. Sci. Total Environ. 2015, 532, 1–13. [Google Scholar] [CrossRef]
- O’Neal, S.T.; Anderson, T.D.; Wu-Smart, J.Y. Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect Sci. 2018, 26, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Pettis, J.S.; Vanengelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Di, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [Green Version]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Tchamitchian, S.; Bonnet, M.; Cousin, M.; Kretzschmar, A.; Brunet, J.-L.; Le Conte, Y. Combined neonicotinoid pesticide and parasite stress alter honeybee queens’ physiology and survival. Sci. Rep. 2016, 6, 31430–31436. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Retschnig, G.; Neumann, P.; Williams, G.R. Thiacloprid-Nosema ceranae interactions in honey bees: Host survivorship but not parasite reproduction is dependent on pesticide dose. J. Invertebr. Pathol. 2014, 118, 18–19. [Google Scholar] [CrossRef]
- Diao, Q.; Li, B.; Zhao, H.; Wu, Y.; Guo, R.; Dai, P.; Chen, D.; Wang, Q.; Hou, C. Enhancement of chronic bee paralysis virus levels in honeybees acute exposed to imidacloprid: A Chinese case study. Sci. Total Environ. 2018, 630, 487–494. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711–20171719. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides ?—A brief review. Environ. Int. 2016, 89–90, 7–11. [Google Scholar] [CrossRef]
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 2016, 6, 32108–32119. [Google Scholar] [CrossRef]
- Tong, L.; Nieh, J.C.; Tosi, S. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere 2019, 237, 12408–12416. [Google Scholar] [CrossRef] [Green Version]
- Foley, K.; Fazio, G.; Jensen, A.B.; Hughes, W.O.H. Nutritional limitation and resistance to opportunistic Aspergillus parasites in honey bee larvae. J. Invertebr. Pathol. 2012, 111, 68–73. [Google Scholar] [CrossRef]
- Koch, H.; Brown, M.J.; Stevenson, P.C. The role of disease in bee foraging ecology. Curr. Opin. Insect Sci. 2017, 21, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolezal, A.G.; Carrillo-Tripp, J.; Judd, T.M.; Allen Miller, W.; Bonning, B.C.; Toth, A.L. Interacting stressors matter: Diet quality and virus infection in honeybee health. R. Soc. Open Sci. 2019, 6, 181803–181813. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Heerman, M.; Peng, W.; Evans, J.D.; Rose, R.; DeGrandi-Hoffman, G.; Simone-Finstrom, M.; Li, J.; Li, Z.; Cook, S.C. The dynamics of deformed wing virus concentration and host defensive gene expression after Varroa mite parasitism in honey bees, Apis mellifera. Insects 2019, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Straub, L.; Williams, G.R.; Vidondo, B.; Khongphinitbunjong, K.; Retschnig, G.; Schneeberger, A.; Chantawannakul, P.; Dietemann, V.; Neumann, P. Neonicotinoids and ectoparasitic mites synergistically impact honeybees. Sci. Rep. 2019, 9, 8159–8168. [Google Scholar] [CrossRef]
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proc. R. Soc. B 2019, 286, 20190331–20190339. [Google Scholar] [CrossRef] [Green Version]
- van Dooremalen, C.; Stam, E.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.; van Langevelde, F.; Blacquière, T. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J. Insect Physiol. 2013, 59, 487–493. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2, 326–332. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Gong, H.-R.; Huang, S.-K.; Sohr, A.; Hu, F.-L.; Chen, Y.P. Evidence of the synergistic interaction of honey bee pathogens Nosema ceranae and deformed wing virus. Vet. Microbiol. 2015, 177, 1–6. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Coulon, M.; Schurr, F.; Martel, A.-C.; Cougoule, N.; Bégaud, A.; Mangoni, P.; Di Prisco, G.; Dalmon, A.; Alaux, C.; Ribière-Chabert, M. Influence of chronic exposure to thiamethoxam and chronic bee paralysis virus on winter honey bees. PLoS ONE 2019, 14, e0220703. [Google Scholar] [CrossRef] [Green Version]
- Coulon, M.; Schurr, F.; Martel, A.-C.; Cougoule, N.; Bégaud, A.; Mangoni, P.; Dalmon, A.; Alaux, C.; Le Conte, Y.; Thiéry, R. Metabolisation of thiamethoxam (a neonicotinoid pesticide) and interaction with the chronic bee paralysis virus in honeybees. Pestic. Biochem. Physiol. 2018, 144, 10–18. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Doublet, V.; Labarussias, M.; De Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef]
- Odemer, R.; Nilles, L.; Linder, N.; Rosenkranz, P. Sublethal effects of clothianidin and Nosema spp. on the longevity and foraging activity of free flying honey bees. Ecotoxicology 2018, 27, 527–538. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.-L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef] [Green Version]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of varroa destructor and sublethal clothianidin doses during the larval stage on subsequent adult honey bee (Apis mellifera L.) health, cellular immunity, deformed wing virus levels and differential gene expression. Microorganisms 2020, 8, 858. [Google Scholar] [CrossRef]
- Reyes-quintana, M.; Espinosa-montaño, L.G.; Prieto-merlos, D.; Koleoglu, G.; Petukhova, T.; Correa-benítez, A.; Guzman-novoa, E. Impact of Varroa destructor and deformed wing virus on emergence, cellular immunity, wing integrity and survivorship of Africanized honey bees in Mexico. J. Invertebr. Pathol. 2019, 164, 43–48. [Google Scholar] [CrossRef]
- Blanken, L.J.; van Langevelde, F.; van Dooremalen, C. Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151738. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [Green Version]
- Almasri, H.; Tavares, D.A.; Diogon, M.; Pioz, M.; Alamil, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Physiological effects of the interaction between Nosema ceranae and sequential and overlapping exposure to glyphosate and difenoconazole in the honey bee Apis mellifera. Ecotoxicol. Environ. Saf. 2021, 217, 112258–112268. [Google Scholar] [CrossRef]
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. J. Invertebr. Pathol. 2018, 159, 78–86. [Google Scholar] [CrossRef] [PubMed]
- van Dooremalen, C.; Cornelissen, B.; Poleij-Hok-Ahin, C.; Blacquière, T. Single and interactive effects of Varroa destructor, Nosema spp., and imidacloprid on honey bee colonies (Apis mellifera). Ecosphere 2018, 9, e02378. [Google Scholar] [CrossRef] [Green Version]
- Porrini, M.P.; Garrido, P.M.; Umpiérrez, M.L.; Porrini, L.P.; Cuniolo, A.; Davyt, B.; González, A.; Eguaras, M.J.; Rossini, C. Effects of synthetic acaricides and Nosema ceranae (microsporidia: Nosematidae) on molecules associated with chemical communication and recognition in honey bee. Vet. Sci. 2020, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.; Wilson, A.E.; Williams, G.R.; Hardy, N.B. Parasites and pesticides act antagonistically on honey bee health. J. Appl. Ecol. 2021, 58, 997–1005. [Google Scholar] [CrossRef]
- Wintermantel, D.; Locke, B.; Andersson, G.K.S.; Semberg, E.; Forsgren, E.; Osterman, J.; Pedersen, T.R.; Bommarco, R.; Smith, H.G.; Rundlöf, M. Field-level clothianidin exposure affects bumblebees but generally not their pathogens. Nat. Commun. 2018, 9, 5446–5455. [Google Scholar] [CrossRef] [Green Version]
- Cutler, G.C.; Scott-Dupree, C.D.; Sultan, M.; McFarlane, A.D.; Brewer, L. A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. PeerJ 2014, 2, e652. [Google Scholar] [CrossRef]
- Rolke, D.; Fuchs, S.; Grünewald, B.; Gao, Z.; Blenau, W. Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: Effects on honey bees (Apis mellifera). Ecotoxicology 2016, 25, 1648–1665. [Google Scholar] [CrossRef] [Green Version]
- Faita, M.R.; Cardozo, M.M.; Amandio, D.T.T.; Orth, A.I.; Nodari, R.O. Glyphosate-based herbicides and Nosema sp. microsporidia reduce honey bee (Apis mellifera L.) survivability under laboratory conditions. J. Apic. Res. 2020, 59, 332–342. [Google Scholar] [CrossRef]
- Gregorc, A.; Silva-Zacarin, E.C.M.; Carvalho, S.M.; Kramberger, D.; Teixeira, E.W.; Malaspina, O. Effects of Nosema ceranae and thiametoxam in Apis mellifera: A comparative study in Africanized and Carniolan honey bees. Chemosphere 2016, 147, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribière-Chabert, M.; Alaux, C.; Thiéry, R.; Le Conte, Y. Interactions between thiamethoxam and deformed wing virus can drastically impair flight behavior of honey bees. Front. Microbiol. 2020, 11, 766–777. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera L.) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, L.; Peghaire, E.; Mone, A.; Diogon, M.; Debroas, D.; Delbac, F.; El Alaoui, H. Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J. Invertebr. Pathol. 2020, 172, 107348–1073456. [Google Scholar] [CrossRef] [PubMed]
- López, J.H.; Krainer, S.; Engert, A.; Schuehly, W.; Riessberger-Gallé, U.; Crailsheim, K. Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Sci. Rep. 2017, 7, 40853–40865. [Google Scholar] [CrossRef] [PubMed]
- Dance, C.; Botías, C.; Goulson, D. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Ecotoxicol. Environ. Saf. 2017, 139, 194–201. [Google Scholar] [CrossRef]
- Renzi, M.T.; Rodríguez-Gasol, N.; Medrzycki, P.; Porrini, C.; Martini, A.; Burgio, G.; Maini, S.; Sgolastra, F. Combined effect of pollen quality and thiamethoxam on hypopharyngeal gland development and protein content in Apis mellifera. Apidologie 2016, 47, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, G.; Simonetto, A.; Carnesecchi, E.; Costa, C.; Hatjina, F.; Tosi, S.; Gilioli, G. Beekeeping and honey bee colony health: A review and conceptualization of beekeeping management practices implemented in Europe. Sci. Total Environ. 2019, 696, 133795–133806. [Google Scholar] [CrossRef]
- Zhang, G.; St. Clair, A.L.; Dolezal, A.; Toth, A.L.; O’Neal, M. Honey bee (hymenoptera: Apidea) pollen forage in a highly cultivated agroecosystem: Limited diet diversity and its relationship to virus resistance. J. Econ. Entomol. 2020, 113, 1062–1072. [Google Scholar] [CrossRef]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Dolezal, A.G.; Clair, A.L.S.; Zhang, G.; Toth, A.L.; O’Neal, M.E. Native habitat mitigates feast–famine conditions faced by honey bees in an agricultural landscape. Proc. Natl. Acad. Sci. USA 2019, 116, 25147–25155. [Google Scholar] [CrossRef] [Green Version]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of nutritional stress on honeybee gut microbiota, immunity, and Nosema ceranae Infection. Microb. Ecol. 2020, 80, 908–919. [Google Scholar] [CrossRef]
- de Jong, E.W.; DeGrandi-Hoffman, G.; Chen, Y.; Graham, H.; Ziolkowski, N. Effects of diets containing different concentrations of pollen and pollen substitutes on physiology, Nosema burden, and virus titers in the honey bee (Apis mellifera L.). Apidologie 2019, 50, 845–858. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Dong, C.; Richardson, L.T.; Donnarumma, F.; Williams, S.T.; Solouki, T.; Murray, K.K. Honey bee proteome responses to plant and cyanobacteria (blue-green algae) diets. ACS Food Sci. Technol. 2020, 1, 17–26. [Google Scholar] [CrossRef]
- Jehlík, T.; Kodrík, D.; Krištůfek, V.; Koubová, J.; Sábová, M.; Danihlík, J.; Tomčala, A.; Čapková Frydrychová, R. Effects of Chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 2019, 50, 564–577. [Google Scholar] [CrossRef]
- Charistos, L.; Parashos, N.; Hatjina, F. Long term effects of a food supplement HiveAliveTM on honey bee colony strength and Nosema ceranae spore counts. J. Apic. Res. 2015, 54, 420–426. [Google Scholar] [CrossRef]
- Alberoni, D.; Baffoni, L.; Gaggìa, F.; Ryan, P.M.; Murphy, K.; Ross, P.R.; Stanton, C.; Di Gioia, D. Impact of beneficial bacteria supplementation on the gut microbiota, colony development and productivity of Apis mellifera L. Benef. Microbes 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Mitton, G.A.; Szawarski, N.; Mitton, F.M.; Iglesias, A.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. Impacts of dietary supplementation with p-coumaric acid and indole-3-acetic acid on survival and biochemical response of honey bees treated with tau-fluvalinate. Ecotoxicol. Environ. Saf. 2020, 189, 109917–109924. [Google Scholar] [CrossRef]
- Szawarski, N.; Saez, A.; Domínguez, E.; Dickson, R.; De Matteis, Á.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I. Effect of abscisic acid (ABA) combined with two different beekeeping nutritional strategies to confront overwintering: Studies on honey bees’ population dynamics and nosemosis. Insects 2019, 10, 329. [Google Scholar] [CrossRef] [Green Version]
- Lamontagne-Drolet, M.; Samson-Robert, O.; Giovenazzo, P.; Fournier, V. The impacts of two protein supplements on commercial honey bee (Apis mellifera L.) colonies. J. Apic. Res. 2019, 58, 800–813. [Google Scholar] [CrossRef]
- Cilia, G.; Fratini, F.; Tafi, E.; Turchi, B.; Mancini, S.; Sagona, S.; Nanetti, A.; Cerri, D.; Felicioli, A. Microbial profile of the ventriculum of honey bee (Apis mellifera ligustica spinola, 1806) fed with veterinary drugs, dietary supplements and non-protein amino acids. Vet. Sci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet. Med. 2021, 190, 105322. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y.; Rivera, R.; Carroll, M.; Chambers, M.; Hidalgo, G.; de Jong, E.W. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie 2016, 47, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Dolasevic, S.; Stevanovic, J.; Aleksic, N.; Glavinic, U.; Deletic, N.; Mladenovic, M.; Stanimirovic, Z. The effect of diet types on some quality characteristics of artificially reared Apis mellifera queens. J. Apic. Res. 2020, 59, 115–123. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Zdybicka-Barabas, A.; Cytryńska, M.; Małek, W. Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol. Res. 2016, 115, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, L.J. Frontiers in effective control of problem parasites in beekeeping. Int. J. Parasitol. Parasites Wildl. 2022, 17, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Toomemaa, K. The synergistic effect of weak oxalic acid and thymol aqueous solutions on Varroa mites and honey bees. J. Apic. Res. 2019, 58, 37–52. [Google Scholar] [CrossRef]
- Adamczyk, S.; Lázaro, R.; Pérez-Arquillué, C.; Conchello, P.; Herrera, A. Evaluation of residues of essential oil components in honey after different anti-Varroa treatments. J. Agric. Food Chem. 2005, 53, 10085–10090. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Xu, S.; Hou, C.; Xu, J.; Zhao, D.; Chen, Y. Antiviral activities of a medicinal plant extract against sacbrood virus in honeybees. Virol. J. 2021, 18, 83–92. [Google Scholar] [CrossRef]
- Tauber, J.P.; Collins, W.R.; Schwarz, R.S.; Chen, Y.; Grubbs, K.; Huang, Q.; Lopez, D.; Peterson, R.; Evans, J.D. Natural product medicines for honey bees: Perspective and protocols. Insects 2019, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.C.V.; Lopes, L.Q.S.; dos Alves, C.F.S.; Fausto, V.P.; Pizzutti, K.; Barboza, V.; de Souza, M.E.; Raffin, R.P.; Gomes, P.; Takamatsu, D.; et al. Antimicrobial activity of tea tree oil nanoparticles against American and European foulbrood diseases agents. J. Asia. Pac. Entomol. 2014, 17, 343–347. [Google Scholar] [CrossRef]
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martín-Hernández, R.; Bernal, J.L.; Bernal, J. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of api-bioxal® and apiherb® treatments against Nosema ceranae infection in Apis mellifera investigated by two qPCR methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Matteo, R.; Lazzeri, L. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms 2021, 9, 949. [Google Scholar] [CrossRef]
- Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Capano, V.; Guerra, I.; Zavatta, L.; Albertazzi, S.; Matteo, R.; Lazzeri, L.; et al. Glucosinolate bioactivation by Apis mellifera workers and its impact on Nosema ceranae infection at the colony level. Biomolecules 2021, 11, 1657. [Google Scholar] [CrossRef]
- Hasan, S. A review on nanoparticles: Their synthesis and types. Res. J. Recent Sci 2015, 2277, 2502–2504. [Google Scholar]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Culha, M.; Kalay, Ş.; Sevim, E.; Pinarbaş, M.; Baş, Y.; Akpinar, R.; Karaoğlu, Ş.A. Biocidal properties of maltose reduced silver nanoparticles against American foulbrood diseases pathogens. Biometals 2017, 30, 893–902. [Google Scholar] [CrossRef]
- Li, J.; Heerman, M.C.; Evans, J.D.; Rose, R.; Li, W.; Rodríguez-García, C.; DeGrandi-Hoffman, G.; Zhao, Y.; Huang, S.; Li, Z.; et al. Pollen reverses decreased lifespan, altered nutritional metabolism and suppressed immunity in honey bees (Apis mellifera) treated with antibiotics. J. Exp. Biol. 2019, 222, jeb202077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masry, S.H.D.; Taha, T.H.; Botros, W.A.; Mahfouz, H.; Al-Kahtani, S.N.; Ansari, M.J.; Hafez, E.E. Antimicrobial activity of camphor tree silver nano-particles against foulbrood diseases and finding out new strain of Serratia marcescens via DGGE-PCR, as a secondary infection on honeybee larvae. Saudi J. Biol. Sci. 2021, 28, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wu, Q.; Long, J.; Lu, B.; Zheng, N.; Hu, C.; Chen, P.; Hu, N.; Lu, C.; Pan, M. Silver nanoparticles are effective in controlling microsporidia. Mater. Sci. Eng. C 2021, 125, 112106–112117. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Li, G.; Zhao, H.; Liu, Z.; Wang, H.; Xu, B.; Guo, X. The wisdom of honeybee defenses against environmental stresses. Front. Microbiol. 2018, 9, 722–736. [Google Scholar] [CrossRef]
- Tang, J.; Ma, C.; Shi, W.; Chen, X.; Liu, Z.; Wang, H. A National survey of managed honey bee colony winter losses (Apis mellifera) in China (2013–2017). Diversity 2020, 12, 318. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Ahmed, Z.H.; Abdel-Rahman, M.F.; Moustafa, A.M. Influence of winter feeding on colony development and the antioxidant system of the honey bee, Apis mellifera. J. Apic. Res. 2020, 59, 752–763. [Google Scholar] [CrossRef]
- Abera, A.; Yakob, H.; Yasin, G. Assessment of production system and constraints of bee keeping practices in damot gale woreda, wolaita zone, southern ethiopia. J. Hortic. For. 2016, 6, 109–114. [Google Scholar]
- Underwood, R.M.; Traver, B.E.; López-Uribe, M.M. Beekeeping management practices are associated with operation size and beekeepers’ philosophy towards in-hive chemicals. Insects 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Seedi, H.R.; Ahmed, H.R.; El-Wahed, A.A.A.; Saeed, A.; Algethami, A.F.; Attia, N.F.; Guo, Z.; Musharraf, S.G.; Khatib, A.; Alsharif, S.M.; et al. Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health. Vet. Sci. 2022, 9, 199. https://doi.org/10.3390/vetsci9050199
El-Seedi HR, Ahmed HR, El-Wahed AAA, Saeed A, Algethami AF, Attia NF, Guo Z, Musharraf SG, Khatib A, Alsharif SM, et al. Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health. Veterinary Sciences. 2022; 9(5):199. https://doi.org/10.3390/vetsci9050199
Chicago/Turabian StyleEl-Seedi, Hesham R., Hanan R. Ahmed, Aida A. Abd El-Wahed, Aamer Saeed, Ahmed F. Algethami, Nour F. Attia, Zhiming Guo, Syed G. Musharraf, Alfi Khatib, Sultan M. Alsharif, and et al. 2022. "Bee Stressors from an Immunological Perspective and Strategies to Improve Bee Health" Veterinary Sciences 9, no. 5: 199. https://doi.org/10.3390/vetsci9050199