Screening the Antioxidant Activity of Thermal or Non-Thermally Treated Fruit Juices by In Vitro and In Vivo Assays
Abstract
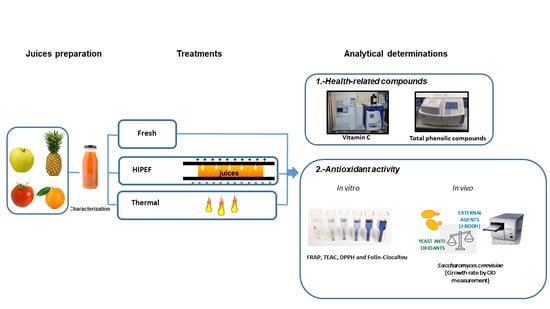
:1. Introduction
2. Materials and Methods
2.1. Juices Preparation
2.2. Pulsed Electric Fields Equipment
2.3. Thermal Treatment
2.4. Packaging and Storage Conditions
2.5. Sample Extraction
2.6. Determination of Health-Related Compounds
2.6.1. Vitamin C
2.6.2. Determination of Phenolic Compounds
2.7. Determination of Antioxidant Activity Using In Vitro Methods
2.8. Determination of Antioxidant Activity Using an In Vivo Method
2.8.1. Yeast Strains, Media and Growth Conditions
2.8.2. Growth Measurements of Treated Cultures
2.9. Statistical Analysis
3. Results and Discussion
3.1. Health-Related Compounds
3.2. Antioxidant Activity
3.2.1. In Vitro Methods
3.2.2. In Vivo Methods
3.3. Relationships between Health-Related Compounds and Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liedhegner, E.A.S.; Gao, X.H.; Micyal, J.J. Mechanisms of altered redox regulation in neurodegenerative disease-Focus on S-glutathionylation. Antioxid. Redox Signal. 2012, 16, 543–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosaka, T.; Tsuji, H.; Tamaoka, A. Biomolecular Modifications Linked to Oxidative Stress in Amyotrophic Lateral Sclerosis: Determining Promising Biomarkers Related to Oxidative Stress. Processes 2021, 9, 1667. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncola, J.; Izakovic, M.; Mazura, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2007, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Meščić Macan, A.; Gazivoda Kraljević, T.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehr, H.A.; Frei, B.; Olofsson, A.M.; Carew, T.E.; Arfors, K.E. Protection from oxidized LDG-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by vitamin C but not vitamin E. Circulation 1995, 91, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Fleuriet, A.; Cheynier, V.; Nicolas, J. Phenolic compounds and oxidative mechanisms in fruits and vegetables. In Phytochemistry of Fruit and Vegetables; Tomás-Barberán, F.A., Robins, R.J., Eds.; Science Publications: Oxford, UK, 1997; pp. 51–85. [Google Scholar]
- Baroni, M.V.; Di Paola Naranjo, R.D.; García-Ferreyra, C.; Otaiza, S.; Wunderlin, D.A. How good antioxidant is the red wine? Comparison of some in vitro and in vivo methods to assess the antioxidant capacity of Argentinian red wines. LWT-Food Sci. Technol. 2012, 47, 1–7. [Google Scholar] [CrossRef]
- Slatnar, A.; Jakopic, J.; Stampar, F.; Veberic, R.; Jamnik, P. The Effect of Bioactive Compounds on In Vitro and In Vivo Antioxidant Activity of Different Berry Juices. PLoS ONE 2012, 7, e47880. [Google Scholar] [CrossRef] [Green Version]
- Stinco, C.M.; Baroni, M.V.; Di Paola Naranjo, R.; Wunderlin, D.A.; Heredia, F.J.; Meléndez-Martínez, A.J.; Vicario, I.M. Hydrophilic antioxidant compounds in orange juice from different fruit cultivars: Composition and antioxidant activity evaluated by chemical and cellular based (Saccharomyces cerevisiae) assays. J. Food Compos. Anal. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Puigpinós, J.; Oms-Oliu, G.; Herrero, E.; Martin-Belloso, O. Antioxidant activity of thermal or non-thermally treated strawberry and mango juices by Saccharomyces cerevisiae growth based assays. LWT-Food Sci. Technol. 2016, 74, 55–61. [Google Scholar] [CrossRef]
- Chew, C.O.; Sanchez-Vega, R.; Mold-Ghazali, H.M.; Mohd-Adzahan, N.M.; Elez-Martinez, P.; Martín-Belloso, O. Inactivation of Listeria innocua in pineapple juice using high intensity pulsed electric field in combination with enzymatic treatment. In IFood 2011; Osnabrueck, Germany, 11 October 2011. [Google Scholar]
- Mosqueda-Melgar, J.; Raybaudi-Massilia, R.M.; Martín-Belloso, O. Microbiological shelf-life and sensory evaluation of fruit juices treated by high-intensity pulsed electric fields and antimicrobials. Food Bioprod. Process. 2012, 90, 205–214. [Google Scholar] [CrossRef]
- Chen, C.S.; Shaw, P.E.; Parish, M.E. Orange and tangerine juices. In Fruit Juice Processing Technology; Nagy, S., Chen, C.S., Shaw, P.E., Eds.; Agscience: Auburndale, FL, USA, 1993; pp. 110–165. [Google Scholar]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative study of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BBvs. Folin-Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrin, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decoloration assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurements of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.M.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Sherman, F. Getting started with yeast. Methods Enzymol. 2002, 350, 3–41. [Google Scholar] [CrossRef]
- Blomberg, A. Measuring growth rate in high-throughput growth phenotyping. Curr. Opin. Biotechnol. 2011, 22, 94–102. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; De Ancos, B.; Cano, M.P. Effect of high-pressure processing on health-promoting attributes of freshly squeezed orange juice (Citrus sinensis L.) during chilled storage. Eur. Food Res. Technol. 2003, 216, 18–22. [Google Scholar] [CrossRef]
- Cámara, M.; Diez, C.; Torija, E. Chemical characterization of pineapple juices and nectars. Principal components analysis. Food Chem. 1995, 54, 93–100. [Google Scholar] [CrossRef]
- Laorko, A.; Tongchitpakdee, S.; Youravong, W. Storage quality of pineapple juice non-thermally pasteurized and clarified by microfiltration. J. Food Eng. 2013, 116, 554–561. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Jin, Z.T.; Ruhlman, K.T.; Qiu, X.; Zhang, Q.H.; Richter, E.R. Microbial safety and shelf-life of apple juice and cider processed by bench and pilot scale PEF systems. Innov. Food Sci. Emerg. Technol. 2000, 1, 77–86. [Google Scholar] [CrossRef]
- Quitão-Teixeira, L.J.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Mota-Ramos, A.; Martín-Belloso, O. Comparative study on antioxidant properties of carrot juice stabilised by high-intensity pulsed electric field or heat treatments. J. Sci. Food Agric. 2009, 89, 2363–2642. [Google Scholar] [CrossRef]
- Elez-Martínez, P.; Martín-Belloso, O. Effects of high intensity pulsed electric field processing conditions on vitamin C and antioxidant capacity of orange juice and gazpacho, a cold vegetable soup. Food Chem. 2007, 102, 201–209. [Google Scholar] [CrossRef]
- Min, S.; Jin, Z.T.; Zhang, Q.H. Commercial scale pulsed electric field processing of tomato juice. J. Agric. Food Chem. 2003, 51, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Persic, M.; Mikulic-Petkovsek, M.; Veberi, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT-Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Agcam, E.; Akyılıdz, G.; Evrendilek, G.A. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurization. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2008, 9, 272–279. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, H.; Flanagan, J.A.; Deemer, E. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, I.M.; Noci, F.; Morgan, D.J.; Cronin, D.A.; Lying, J.G. The effect of pulsed electric fields, ultraviolet light or high intensity light pulses in combination with manothermosonication on selected physico-chemical and sensory attributes of an orange and carrot juice blend. Food Bioprod. Process. 2012, 90, 442–448. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS+ Radical Cation Assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of two cooking procedures on phytochemical compounds, total antioxidant capacity and colour of selected frozen vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.A.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Amari, F.; Fettouche, A.; Samra, M.A.; Kefalas, P.; Kampranis, S.C.; Makris, A.M. Antioxidant small molecules confer variable protection against oxidative damage in yeast mutants. J. Agric. Food Chem. 2008, 56, 11740–11751. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bartosz, G. Ascorbate and thiol antioxidants abolish sensitivity of yeast Saccharomyces cerevisiae to disulfiram. Cell Biol. Toxicol. 2012, 28, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, G.; Horta, B.B.; Pimenta, D.C.; Augusto, O.; Netto, L.E.S. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc. Natl. Acad. Sci. USA 2007, 104, 4886–4891. [Google Scholar] [CrossRef] [PubMed] [Green Version]

 ) and thermally-treated juices (
) and thermally-treated juices (  ).
).
 ) and thermally-treated juices (
) and thermally-treated juices (  ).
).
| Parameters a | Tomato Juice | Apple Juice | Pineapple Juice | Orange Juice |
|---|---|---|---|---|
| Soluble solids (°Brix) | 4.65 ± 0.13 | 10.82 ± 0.09 | 13.51 ± 0.05 | 13.56 ± 0.07 |
| pH | 4.23 ± 0.01 | 4.28 ± 0.03 | 3.56 ± 0.02 | 3.56 ± 0.01 |
| Colour | ||||
| L* | 23.21 ± 0.04 | 25.32 ± 0.01 | 60.35 ± 0.07 | 40.25 ± 0.12 |
| a* | 6.83 ± 0.17 | 0.41 ± 0.09 | −3.20 ± 0.11 | 6.83 ± 0.13 |
| b* | 5.21 ± 0.09 | 3.85 ± 0.04 | 20.31 ± 0.07 | 27.52 ± 0.15 |
| Electrical conductivity (S/m) | 0.63 ± 0.21 | 0.21 ± 0.02 | 0.36 ± 0.11 | 0.38 ± 0.05 |
| Parameters | Treatments | Tomato Juice * | Apple Juice * | Pineapple Juice * | Orange Juice * |
|---|---|---|---|---|---|
| Vitamin C (mg of ascorbic acid/L of juice) | Fresh | 128 ± 5 aC | 10 ± 1 aD | 231 ± 13 aB | 344 ± 2 aA |
| HIPEF | 110 ± 1 bC | 12 ± 3 aD | 145 ± 4 cB | 276 ± 3 cA | |
| TT | 102 ± 2 cC | 11 ± 1 aD | 160 ± 5 bB | 291 ± 4 bA | |
| Total phenolic compounds (mg of gallic acid/L of juice) | Fresh | 167 ± 2 aD | 547 ± 5 aB | 276 ± 7 aC | 1238 ± 49 aA |
| HIPEF | 154 ± 1 bD | 551 ± 12 aB | 280 ± 11 aC | 1185 ± 58 aA | |
| TT | 149 ± 1 cD | 546 ± 14 aB | 247 ± 15 bC | 1190 ± 68 aA |
| Methods | Treatments | Antioxidant Activity of Juices (mmol of TROLOX/L of Juice) * | |||
|---|---|---|---|---|---|
| Tomato | Apple | Pineapple | Orange | ||
| DPPH | Fresh | 1.44 ± 0.11 aC | 0.68 ± 0.09 aD | 3.70 ± 0.06 bA | 2.15 ± 0.10 aB |
| HIPEF | 1.38 ± 0.06 aC | 0.41 ± 0.12 bD | 3.33 ± 0.05 cA | 1.87 ± 0.11 bB | |
| TT | 1.44 ± 0.04 aC | 0.45 ± 0.03 bD | 4.04 ± 0.15 aA | 2.26 ± 0.16 aB | |
| TEAC | Fresh | 1.74 ± 0.09 aD | 4.73 ± 0.03 aB | 3.39 ± 0.02 bC | 6.58 ± 0.02 bA |
| HIPEF | 1.83 ± 0.03 aD | 4.69 ± 0.08 aB | 3.46 ± 0.20 bC | 6.63 ± 0.14 bA | |
| TT | 1.84 ± 0.04 aD | 4.78 ± 0.16 aB | 3.91 ± 0.18 aC | 7.07 ± 0.56 aA | |
| FRAP | Fresh | 0.138 ± 0.009 aC | 0.135 ± 0.044 aC | 0.609 ± 0.014 bB | 0.904 ± 0.045 aA |
| HIPEF | 0.135 ± 0.001 aC | 0.119 ± 0.040 aC | 0.602 ± 0.012 bB | 0.910 ± 0.047 aA | |
| TT | 0.135 ± 0.002 aC | 0.138 ± 0.035 aC | 0.740 ± 0.038 aA | 0.656 ± 0.002 bB | |
| Folin-Ciocalteu | Fresh | 3.38 ± 0.15 aB | 3.30 ± 0.27 aB | 10.79 ± 0.52 aA | 12.01 ± 1.11 aA |
| HIPEF | 3.26 ± 0.11 aB | 3.23 ± 0.21 aB | 9.80 ± 0.35 bA | 10.46 ± 0.94 bA | |
| TT | 3.28 ± 0.01 aC | 3.76 ± 1.20 aC | 8.66 ± 0.09 cB | 10.48 ± 1.64 bA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odriozola-Serrano, I.; Bellí, G.; Puigpinós, J.; Herrero, E.; Martín-Belloso, O. Screening the Antioxidant Activity of Thermal or Non-Thermally Treated Fruit Juices by In Vitro and In Vivo Assays. Beverages 2022, 8, 36. https://doi.org/10.3390/beverages8020036
Odriozola-Serrano I, Bellí G, Puigpinós J, Herrero E, Martín-Belloso O. Screening the Antioxidant Activity of Thermal or Non-Thermally Treated Fruit Juices by In Vitro and In Vivo Assays. Beverages. 2022; 8(2):36. https://doi.org/10.3390/beverages8020036
Chicago/Turabian StyleOdriozola-Serrano, Isabel, Gemma Bellí, Judit Puigpinós, Enric Herrero, and Olga Martín-Belloso. 2022. "Screening the Antioxidant Activity of Thermal or Non-Thermally Treated Fruit Juices by In Vitro and In Vivo Assays" Beverages 8, no. 2: 36. https://doi.org/10.3390/beverages8020036