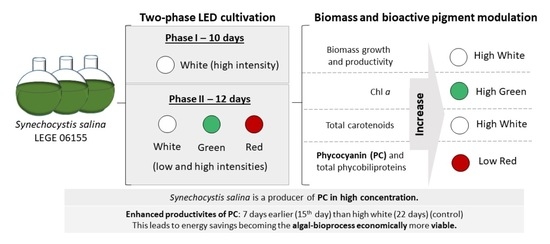
Light Modulation for Bioactive Pigment Production in Synechocystis salina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Cyanobacterial Cultivation Conditions
2.3. Experimental Design
2.4. Determination of Cyanobacterial Biomass Growth and Production
2.5. In Vivo Chlorophyll a Fluorescence Monitoring
2.6. Pigment Extraction and Quantification
2.6.1. Pigment Extract Preparation
2.6.2. Determination of Pigment Content
2.7. Carotenoid Profile and Quantification
2.8. Statistical Analysis
3. Results
3.1. Growth and Biomass Production
3.2. Photosynthetic Efficiency
3.3. Effect of Light Treatment on Pigment Content and Profile
3.3.1. General Pigment Content
3.3.2. Carotenoid Profile
3.4. PC/PE Ratio
3.5. Pigment Productivities
4. Discussion
4.1. Biomass Production and Photosynthetic Efficiency
4.2. Acclimatization and Pigment Content and Profile
4.3. Pigment Productivities and Biotechnological Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khajepour, F.; Hosseini, S.A.; Ghorbani Nasrabadi, R.; Markou, G. Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl. Biochem. Biotechnol. 2015, 176, 2279–2289. [Google Scholar] [CrossRef]
- Schagerl, M.; Müller, B. Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. J. Plant Physiol. 2006, 163, 709–716. [Google Scholar] [CrossRef]
- Porra, R.J.; Pfundel, E.E.; Endel, N. Metabolism and function of photosynthetic pigments. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO Publishing: Paris, France, 1997; pp. 85–126. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Chanu, N.K.; Chaurasia, N. Cyanobacterial pigments and their fluorescence characteristics: Applications in research and industry. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: New York, NY, USA, 2020; pp. 55–72. [Google Scholar]
- Kehoe, D.M. Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 9029–9030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.K.; Sonani, R.R.; Prasad Rastogi, R.; Madamwar, D. The phycobilisomes: An early requisite for efficient photosynthesis in cyanobacteria. EXCLI J. 2015, 14, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Gutu, A.; Kehoe, D.M. Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol. Plant 2012, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Brunet, C.; Johnsen, G.; Lavaud, J.; Roy, S. Pigments and photoacclimation processes. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Roy, S., Llewellyn, C.A., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef]
- Ojit, S.K.; Indrama, T.; Gunapati, O.; Avijeet, S.O.; Subhalaxmi, S.A.; Silvia, C.; Indira, D.W.; Romi, K.; Thadoi, D.A.; Tiwari, O.N.; et al. The response of phycobiliproteins to light qualities in Anabaena circinalis. J. Appl. Biol. Biotechnol. 2015, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Lau, N.; Matsui, M.; Abdullah, A.A. Cyanobacteria: Photoautotrophic microbial factories for the sustainable synthesis of industrial products. Biomed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Cyanobacterial pigments: Photosynthetic function and biotechnological purposes. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Elsevier Academic Press: London, UK, 2022; ISBN 9780128214916. [Google Scholar]
- Milia, M.; Corrias, F.; Addis, P.; Zitelli, G.C.; Cicchi, B.; Torzillo, G.; Andreotti, V.; Angioni, A. Influence of Different Light Sources on the Biochemical Composition of Arthrospira spp. Grown in Model Systems. Foods 2022, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Niangoran, N.U.F.; Buso, D.; Zissis, G.; Prudhomme, T. Influence of light intensity and photoperiod on energy efficiency of biomass and pigment production of Spirulina (Arthrospira platensis). OCL 2021, 28, 37. [Google Scholar] [CrossRef]
- Assunção, J.; Malcata, F.X. Enclosed “non-conventional” photobioreactors for microalga production: A review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Glemser, M.; Heining, M.; Schmidt, J.; Becker, A.; Garbe, D.; Buchholz, R.; Brück, T. Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: Current state and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1077–1088. [Google Scholar] [CrossRef]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A.C. White and red LEDs as two-phase batch for cyanobacterial pigments production. Bioresour. Technol. 2020, 307, 123105. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Synechocystis salina: Potential bioactivity and combined extraction of added-value metabolites. J. Appl. Phycol. 2021, 33, 3731–3746. [Google Scholar] [CrossRef]
- Afonso, T.B.; Costa, M.S.; Rezende De Castro, R.; Freitas, S.; Silva, A.; Schneider, M.P.C.; Martins, R.; Leão, P.N. Bartolosides E-K from a Marine coccoid cyanobacterium. J. Nat. Prod. 2016, 79, 2504–2513. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Bland, E.; Angenent, L.T. Pigment-targeted light wavelength and intensity promotes efficient photoautotrophic growth of Cyanobacteria. Bioresour. Technol. 2016, 216, 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pojidaeva, E.; Zinchenko, V.; Shestakov, S.V.; Sokolenko, A. Involvement of the SppA1 peptidase in acclimation to saturating light intensities in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2004, 186, 3991–3999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Montgomery, B.L. Mechanisms and fitness implications of photomorphogenesis during chromatic acclimation in cyanobacteria. J. Exp. Bot. 2016, 67, 4079–4090. [Google Scholar] [CrossRef]
- Palenik, B. Chromatic Adaptation in Marine. Microbiology 2001, 67, 991–994. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Jerez, C.G.; Korbee, N. Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat. Am. J. Aquat. Res. 2013, 41, 801–819. [Google Scholar] [CrossRef]
- Pagels, F.; Barufi, J.B.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light regulating metabolic responses of cyanobium sp. (cyanobacteria). Fundam. Appl. Limnol. 2020, 193, 285–297. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Johnsen, G.; Sakshaug, E. Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulseamplitude-modulated and fast-repetition-rate fluorometry. J. Phycol. 2007, 43, 1236–1251. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1994, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Cordara, A.; Re, A.; Pagliano, C.; Van Alphen, P.; Pirone, R.; Saracco, G.; dos Santos, F.B.; Hellingwerf, K.; Vasile, N. Analysis of the light intensity dependence of the growth of Synechocystis and of the light distribution in a photobioreactor energized by 635 nm light. PeerJ 2018, 6, e5256. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.B.; Wu, J.Y.; Wang, C.F.; Fu, C.C.; Shieh, C.J.; Chen, C.I.; Wang, C.Y.; Liu, Y.C. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Pagels, F.; Bonomi-Barufi, J.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light quality triggers biochemical modulation of Cyanobium sp.—photobiology as tool for biotechnological optimization. J. Appl. Phycol. 2020, 32, 2851–2861. [Google Scholar] [CrossRef]
- Da Fontoura Prates, D.; Radmann, E.M.; Duarte, J.H.; de Morais, M.G.; Costa, J.A.V. Spirulina cultivated under different light emitting diodes: Enhanced cell growth and phycocyanin production. Bioresour. Technol. 2018, 256, 38–43. [Google Scholar] [CrossRef]
- Tian, F.; Buso, D.; Wang, T.; Lopes, M.; Niangoran, U.; Zissis, G. Effect of Red and Blue LEDs on the Production of Phycocyanin by Spirulina Platensis Based on Photosynthetically Active Radiation. J. Sci. Technol. Light. 2018, 41, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Kirilovsky, D. Changing Color for Photoprotection: The Orange Carotenoid Protein. Trends Plant Sci. 2020, 25, 92–104. [Google Scholar] [CrossRef]
- Sedoud, A.; López-Igual, R.; Rehman, A.U.; Wilson, A.; Perreau, F.; Boulay, C.; Vass, I.; Krieger-Liszkay, A.; Kirilovsky, D. The cyanobacterial photoactive orange carotenoid protein is an excellent singlet oxygen quencher. Plant Cell 2014, 26, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbunov, M.Y.; Kuzminov, F.I.; Fadeev, V.V.; Kim, J.D.; Falkowski, P.G. A kinetic model of non-photochemical quenching in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 1591–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravelonandro, P.H.; Ratianarivo, D.H.; Joannis-cassan, C. Influence of light quality and intensity in the cultivation of Spirulina platensis from Toliara (Madagascar) in a closed system. J. Chem. Tecnol. Biotechnol. 2008, 848, 842–848. [Google Scholar] [CrossRef]
- Paerl, H.W. Cyanobacterial carotenoids: Their roles in maintaining optimal photosynthetic production among aquatic bloom forming genera. Oecologia 1984, 61, 143–149. [Google Scholar] [CrossRef]
- Kehoe, D.M.; Gutu, A. Responding to color: The regulation of complementary chromatic adaptation. Annu. Rev. Plant Biol. 2006, 57, 127–150. [Google Scholar] [CrossRef]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum—A prospective phycobiliprotein-producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef]
- Mattos, E.R.; Singh, M.; Cabrera, M.L.; Das, K.C. Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 2015, 81, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Maurya, R.R.; Patidar, S.K.; Haldar, S.; Mishra, S. Effect of light quality on the C-phycoerythrin production in marine cyanobacteria Pseudanabaena sp. isolated from Gujarat coast, India. Protein Expr. Purif. 2012, 81, 5–10. [Google Scholar] [CrossRef]
- Rivera, C.; Niño, L.; Gelves, G. Modeling of phycocyanin production from Spirulina platensis using different light-emitting diodes. S. Afr. J. Chem. Eng. 2021, 37, 167–178. [Google Scholar] [CrossRef]
- Keithellakpam, O.S.; Nath, T.O.; Oinam, A.S.; Thingujam, I.; Oinam, G.; Dutt, S.G. Effect of external pH on cyanobacterial phycobiliproteins production and ammonium excretion. J. Appl. Biol. Biotechnol. 2015, 3, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.M.; Kumar, K.; Das, D. Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour. Technol. 2014, 166, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Fu, C.C.; Liu, Y.C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Van Hieu, H.; Quang-Tuong, L.; Cong Doan, B. The Production of High Phycocyanin by Applications of Red Light-Emitting Diodes (LEDs) In Vitro Algae Growth on Spirulina platensis. J. Nano-Electron. Phys. 2021, 13, 03034. [Google Scholar] [CrossRef]

 ); green (
); green (  ) and red (
) and red (  ).
).
 ); green (
); green (  ) and red (
) and red (  ).
).



| Light Treatments | µaverage (d−1) | Px (g·L−1·d−1) |
|---|---|---|
| W (control) | 0.015 ± 0.00 a | 0.18 ± 0.00 a |
| LW | 0.014 ± 0.01 a | 0.17 ± 0.00 a |
| G | 0.000 ± 0.00 b | 0.13 ± 0.00 b |
| LG | 0.000 ± 0.00 b | 0.13 ± 0.00 b |
| R | 0.012 ± 0.00 a | 0.16 ± 0.00 a |
| LR | 0.011 ± 0.00 a,c | 0.15 ± 0.00 a,c |
| Identified Pigments | Concentration of Pigment (µg·gDW−1) | ||||||
|---|---|---|---|---|---|---|---|
| W (T10) | W | LW | G | LG | R | LR | |
| Lycopene * | 0.86 ± 0.04 a | 2.19 ± 0.02 b | 0.82 ± 0.27 a | 1.33 ± 0.05 c | 0.89 ± 0.15 a,d | 2.30 ± 0.16 b | 1.02 ± 0.14 c |
| Zeaxanthin | 3.36 ± 0.24 a | 14.60 ± 0.52 b | 3.87 ± 0.65 a | 6.43 ± 0.17 c | 5.14 ± 0.06 c | 5.34 ± 0.54 c | 6.19 ± 0.22 c |
| Echinenone | 0.49 ± 0.02 a | 1.45 ± 0.18 b | 0.51 ± 0.01 a | 0.94 ± 0.01 c | 0.78 ± 0.03 d | 0.76 ± 0.04 d | 0.71 ± 0.07 d |
| β-carotene | 2.86 ± 0.02 a | 11.34 ± 0.70 b | 3.71 ± 0.37 a | 5.57 ± 0.44 c | 4.66 ± 0.22 c | 3.95 ± 0.17 a | 5.39 ± 0.46 c |
| α-carotene | 0.56 ± 0.03 a | 1.52 ± 0.14 b | 0.63 ± 0.16 a | 0.82 ± 0.05 c | 0.79 ± 0.04 c | 0.61 ± 0.01 a | 0.68 ± 0.02 a |
| PC/PE Ratio (%) | ||||
|---|---|---|---|---|
| Light Treatment | Day 10 (Phase I) | Light Treatments | Day 15 (Phase II) | Day 22 (Phase II) |
| W (control) | 60/40 ± 2.5 a | W (control) | 79/21 ± 3.2 b | 76/24 ± 2.6 b |
| LW | 61/39 ± 3.9 a | 70/30 ± 3.2 b | ||
| G | 69/31 ± 2.8 b | 68/32 ± 4.2 b | ||
| LG | 64/36 ± 3.4 a | 44/56 ± 2.2 b | ||
| R | 77/23 ± 1.1 b | 76/24 ± 3.1 b | ||
| LR | 82/18 ± 2.0 b | 85/15 ± 0.5 b | ||
| Maximum Pigment Productivities (mg·g−1·L−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light Treatment | Chl a (mg·g−1·L−1) | Day | Total Carotenoids (mg·g−1·L−1) | Day | Phycocyanin (mg·g−1·L−1) | Day | Allophycocyanin (mg·g−1·L−1) | Day | Phycoerythrin (mg·g−1·L−1) | Day | Total PBPs (mg·g−1 L−1) | Day |
| W (control) | 1.03 ± 0.02 a | 6 | 0.32 ± 0.01 a | 15 | 1.42 ± 0.13 a | 22 | 0.33 ± 0.01 a | 6 | 0.27 ± 0.01 a | 22 | 1.52 ± 0.02 a | 22 |
| LW | 0.59 ± 0.03 b | 13 | 0.13 ± 0.0 b | 22 | 0.08 ± 0.00 b | 15 | 0.07 ± 0.01 b | 20 | 0.08 ± 0.01 b | 20 | 0.25 ± 0.04 b | 18 |
| G | 0.96 ± 0.00 c | 13 | 0.29 ± 0.00 c | 13 | 0.51 ± 0.02 c | 18 | 0.09 ± 0.00 b | 22 | 0.12 ± 0.01 c | 18 | 0.65 ± 0.03 c | 18 |
| LG | 0.72 ± 0.06 d | 11 | 0.13 ± 0.01 b | 22 | 0.19 ± 0.0 d | 18 | 0.11 ± 0.01 b,c | 20 | 0.11 ± 0.01 c | 15 | 0.40 ± 0.03 d | 18 |
| R | 0.59 ± 0.00 b | 15 | 0.19 ± 0.00 d | 15 | 1.29 ± 0.39 a | 15 | 0.21 ± 0.01 d | 15 | 0.27 ± 0.01 a | 15 | 1.70 ± 0.07 e | 15 |
| LR | 0.75 ± 0.00 d | 15 | 0.23 ± 0.02 e | 13 | 1.30 ± 0.04 a | 15 | 0.27 ± 0.02 e | 18 | 0.21 ± 0.00 d | 18 | 1.43 ± 0.03 a | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, J.; Pagels, F.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Light Modulation for Bioactive Pigment Production in Synechocystis salina. Bioengineering 2022, 9, 331. https://doi.org/10.3390/bioengineering9070331
Assunção J, Pagels F, Tavares T, Malcata FX, Guedes AC. Light Modulation for Bioactive Pigment Production in Synechocystis salina. Bioengineering. 2022; 9(7):331. https://doi.org/10.3390/bioengineering9070331
Chicago/Turabian StyleAssunção, Joana, Fernando Pagels, Tânia Tavares, F. Xavier Malcata, and A. Catarina Guedes. 2022. "Light Modulation for Bioactive Pigment Production in Synechocystis salina" Bioengineering 9, no. 7: 331. https://doi.org/10.3390/bioengineering9070331