Strategy for Managing Industrial Anaerobic Sludge through the Heterotrophic Cultivation of Chlorella sorokiniana: Effect of Iron Addition on Biomass and Lipid Production
Abstract
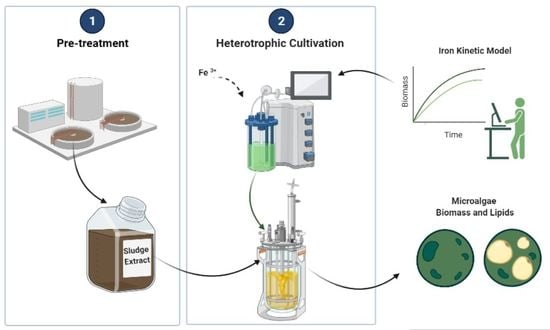
:1. Introduction
2. Materials and Methods
2.1. The Heterotrophic Cultivation of C. sorokiniana
2.2. Quantification of Respiratory Activity in Shake Flasks
2.3. Evaluation of the Iron Effect on Heterotrophic Cultivation
2.4. Kinetic Model for Iron Co-Limitation Effect
2.5. Use of Anaerobically Digested Waste Sludge Extract as a Media Supplement for C. sorokiniana Cultivation under Heterotrophic Conditions
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quantification of Respiratory Activity in Shake Flasks
3.2. Evaluation of the Iron Effect on Heterotrophic Cultivation
3.3. Kinetic Model for Iron Co-Limitation Effect
3.4. Use of Anaerobically Digested Waste Sludge Extract as a Media Supplement for C. sorokiniana Cultivation under Heterotrophic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guldhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic cultivation of microalgae using aquaculture wastewater: A biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Shi, S.; Xu, G.; Yu, H.; Zhang, Z. Strategies of valorization of sludge from wastewater treatment. J. Chem. Technol. Biotechnol. 2018, 93, 936–944. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, T.; Yu, X.; Bates, P.D.; Dong, T.; Chen, S. High-density fed-batch culture of a thermotolerant microalga chlorella sorokiniana for biofuel production. Appl. Energy 2013, 108, 281–287. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cao, L.; Gao, C.; Chen, Y.; Zhang, T.C. Characteristics of wastewater treatment by Chlorella sorokiniana and comparison with activated sludge. Water Sci. Technol. 2019, 80, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Kuo, E.-W.; Nagarajan, D.; Ho, S.-H.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef] [PubMed]
- Morgese, M.G.; Mhillaj, E.; Francavilla, M.; Bove, M.; Morgano, L.; Tucci, P.; Trabace, L.; Schiavone, S. Chlorella sorokiniana Extract Improves Short-Term Memory in Rats. Molecules. 2016, 21, 1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.F.; Liu, J.J.; Chauhan, A.S.; Hu, H.; Ma, L.L.; Lam, P.K.S.; Zeng, R.J. Combining nitrogen starvation with sufficient phosphorus supply for enhanced biodiesel productivity of Chlorella vulgaris fed on acetate. Algal Res. 2016, 17, 261–267. [Google Scholar] [CrossRef]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Sun, X.; Lu, Q. Cultivation of energy microalga Chlorella vulgaris with low–toxic sludge extract. Water Sci. Technol. 2021, 83, 818–830. [Google Scholar] [CrossRef]
- Tan, X.-B.; Meng, J.; Tang, Z.; Yang, L.-B.; Zhang, W.-W. Optimization of algae mixotrophic culture for nutrients recycling and biomass/lipids production in anaerobically digested waste sludge by various organic acids addition. Chemosphere 2020, 244, 125509. [Google Scholar] [CrossRef] [PubMed]
- Prathna, T.C.; Srivastava, A. Ferric chloride for odour control: Studies from wastewater treatment plants in India. Water Pract. Technol. 2020, 16, 35–41. [Google Scholar] [CrossRef]
- Singh, P.; Guldhe, A.; Kumari, S.; Rawat, I.; Bux, F. Combined metals and EDTA control: An integrated and scalable lipid enhancement strategy to alleviate biomass constraints in microalgae under nitrogen limited conditions. Energy Convers. Manag. 2016, 114, 100–109. [Google Scholar] [CrossRef]
- Wan, M.; Jin, X.; Xia, J.; Rosenberg, J.N.; Yu, G.; Nie, Z.; Oyler, G.A.; Betenbaugh, M.J. The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2014, 98, 9473–9481. [Google Scholar] [CrossRef]
- Glaesener, A.G.; Merchant, S.S.; Blaby-Haas, C.E. Iron economy in Chlamydomonas reinhardtii. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concas, A.; Steriti, A.; Pisu, M.; Cao, G. Comprehensive modeling and investigation of the effect of iron on the growth rate and lipid accumulation of Chlorella vulgaris cultured in batch photobioreactors. Bioresour. Technol. 2014, 153, 340–350. [Google Scholar] [CrossRef]
- Ojo, E.; Auta, H.; Baganz, F.; Lye, G. Development of pH stable media for phototrophic and heterotrophic cultivation of Chlorella sorokiniana. In Proceedings of the Poster Session Presented at 6th European Phycology Conference, London, UK, 23–28 August 2015. [Google Scholar]
- Liu, Z.-Y.; Wang, G.-C.; Zhou, B.-C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef]
- Ren, H.Y.; Liu, B.F.; Kong, F.; Zhao, L.; Xie, G.J.; Ren, N.Q. Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour. Technol. 2014, 169, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cheng, J.J. Effects of Selenite on Unicellular Green Microalga Chlorella pyrenoidosa: Bioaccumulation of Selenium, Enhancement of Photosynthetic Pigments, and Amino Acid Production. J. Agric. Food Chem. 2017, 65, 10875–10883. [Google Scholar] [CrossRef]
- Battah, M.; El-Ayoty, Y.; Abomohra, A.E.-F.; El-Ghany, S.A.; Esmael, A. Effect of Mn2+, Co2+ and H2O2 on biomass and lipids of the green microalga Chlorella vulgaris as a potential candidate for biodiesel production. Ann. Microbiol. 2015, 65, 155–162. [Google Scholar] [CrossRef]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019, 39, 101432. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Klöckner, W.; Gacem, R.; Anderlei, T.; Raven, N.; Schillberg, S.; Lattermann, C.; Büchs, J. Correlation between mass transfer coefficient kLa and relevant operating parameters in cylindrical disposable shaken bioreactors on a bench-to-pilot scale. J. Biol. Eng. 2013, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Anderlei, T.; Zang, W.; Papaspyrou, M.; Büchs, J. Online respiration activity measurement (OTR, CTR, RQ) in shake flasks. Biochem. Eng. J. 2004, 17, 187–194. [Google Scholar] [CrossRef]
- Cardenas-Fernandez, M.; Caicedo-Ortega, N.; Villegas-Torres, M.; Baganz, F. Enzymatic cell wall disruption of Chlorella sorokiniana grown under heterotrophic conditions for sustainable biodiesel production. In Proceedings of the Poster Session Presented at the International Conference on Algal Biomass, Biofuels, and Bioproducts, Seattle, WA, USA, 11–13 June 2018. [Google Scholar]
- Huang, A.; Sun, L.; Wu, S.; Liu, C.; Zhao, P.; Xie, X.; Wang, G. Utilization of glucose and acetate by Chlorella and the effect of multiple factors on cell composition. J. Appl. Phycol. 2017, 29, 23–33. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; De Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Ermis, H.; Guven-Gulhan, U.; Cakir, T.; Altinbas, M. Effect of iron and magnesium addition on population dynamics and high value product of microalgae grown in anaerobic liquid digestate. Sci. Rep. 2020, 10, 3510. [Google Scholar] [CrossRef] [Green Version]
- Krichen, E.; Rapaport, A.; Le Floc’h, E.; Fouilland, E. Demonstration of facilitation between microalgae to face environmental stress. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, W.Y.; Ling, T.C.; Show, P.L.; Juan, J.C.; Chang, J.-S.; Lee, D.-J. Cultivation in wastewaters for energy: A microalgae platform. Appl. Energy 2016, 179, 609–625. [Google Scholar] [CrossRef]
- Ghidossi, T.; Marison, I.; Devery, R.; Gaffney, D.; Forde, C. Characterization and Optimization of a Fermentation Process for the Production of High Cell Densities and Lipids Using Heterotrophic Cultivation of Chlorella protothecoides. Ind. Biotechnol. 2017, 13, 253–259. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Kassim, K.A.; Shokravi, Z.; Jakarni, F.M.; Liu, H.Y.; Zaini, N.; Tan, L.S.; Islam, A.B.M.S.; Shokravi, H. Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: A review. Renew. Sustain. Energy Rev. 2020, 119, 109621. [Google Scholar] [CrossRef]





| Treatment | Carbon Source (g L−1) | |
|---|---|---|
| Acetate | Glucose | |
| E1 | 0 | 5 |
| E2 | 0 | 10 |
| E3 | 5 | 5 |
| E4 | 5 | 10 |
| Parameter | Unit | C. sorokiniana | Scenedesmus sp. |
|---|---|---|---|
| µFe | h−1 | 0.58 | 0.105 |
| µmax | h−1 | 0.256 | 0.085 |
| Yx/s | g Biomass g Glucose−1 | 0.525 | 0.23 |
| Ks | g Glucose L−1 | 5.33 | 12.56 |
| KFe | mg Fe L−1 | 12 | 0.0124 |
| KiFe | mg Fe L−1 | 74 | 52 |
| n | 1 | 1.435 |
| Elemental Analysis | Concentration (g L−1) | |
|---|---|---|
| Anaerobic Waste Sludge | Anaerobic Sludge Extract | |
| Total organic carbon (TOC.) | 6.550 | 1.178 |
| Total nitrogen (TN) | 9.510 | 0.251 |
| Iron (Fe) | 11.670 | 0.060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charria-Girón, E.; Amazo, V.; De Angulo, D.; Hidalgo, E.; Villegas-Torres, M.F.; Baganz, F.; Caicedo Ortega, N.H. Strategy for Managing Industrial Anaerobic Sludge through the Heterotrophic Cultivation of Chlorella sorokiniana: Effect of Iron Addition on Biomass and Lipid Production. Bioengineering 2021, 8, 82. https://doi.org/10.3390/bioengineering8060082
Charria-Girón E, Amazo V, De Angulo D, Hidalgo E, Villegas-Torres MF, Baganz F, Caicedo Ortega NH. Strategy for Managing Industrial Anaerobic Sludge through the Heterotrophic Cultivation of Chlorella sorokiniana: Effect of Iron Addition on Biomass and Lipid Production. Bioengineering. 2021; 8(6):82. https://doi.org/10.3390/bioengineering8060082
Chicago/Turabian StyleCharria-Girón, Esteban, Vanessa Amazo, Daniela De Angulo, Eliana Hidalgo, María Francisca Villegas-Torres, Frank Baganz, and Nelson. H. Caicedo Ortega. 2021. "Strategy for Managing Industrial Anaerobic Sludge through the Heterotrophic Cultivation of Chlorella sorokiniana: Effect of Iron Addition on Biomass and Lipid Production" Bioengineering 8, no. 6: 82. https://doi.org/10.3390/bioengineering8060082