Rheological Behavior of High Cell Density Pseudomonas putida LS46 Cultures during Production of Medium Chain Length Polyhydroxyalkanoate (PHA) Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Organism, Medium, and Substrate
2.2. Reactor Setup and Operation
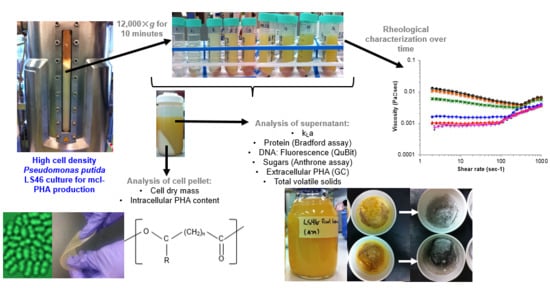
2.3. Sample Treatment
2.4. Viscosity Measurements (Pilot Scale)
2.5. Off-Line Measurement of the Volumetric Oxygen Mass Transfer Coefficient (Bench-Scale)
2.6. Analysis of Organic Products in the Supernatant (Bench-Scale)
3. Results and Discussion
3.1. Growth and mcl-PHA Synthesis
3.2. Rheological Characterization of the Cultivation Medium
3.3. Quantifying Components of the Extracellular Matrix
3.4. Engineering Significance: Effects on Oxygen Transfer Rate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hale, R.C. Are the Risks from Microplastics Truly Trivial? Environ. Sci. Technol. 2018, 52, 931. [Google Scholar] [CrossRef]
- Harding, K.; Dennis, J.; Vonblottnitz, H.; Harrison, S. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef]
- Masood, F.; Yasin, T.; Hameed, A. Polyhydroxyalkanoates—What are the uses? Current challenges and perspectives. Crit. Rev. Biotechnol. 2015, 35, 514–521. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxyalkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, I. Strategies for large-scale production of polyhydroxyalkanoates. Chem. Biochem. Eng. Q. 2015, 29, 157–172. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef]
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phospate limitation. Biotechnol. Bioeng. 1997, 55, 28–32. [Google Scholar] [CrossRef]
- Shang, L.; Jiang, M.; Chang, H.N. Poly(3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol. Lett. 2003, 25, 1415–1419. [Google Scholar] [CrossRef]
- Wang, F.; Lee, S.Y. Poly(3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 1997, 63, 3703–3706. [Google Scholar]
- Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Blunt, W.; Levin, D.; Cicek, N. Bioreactor Operating Strategies for Improved Polyhydroxyalkanoate (PHA) Productivity. Polymers 2018, 10, 1197. [Google Scholar] [CrossRef]
- Riesenberg, D.; Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 1999, 51, 422–430. [Google Scholar] [CrossRef]
- Gabelle, J.C.; Augier, F.; Carvalho, A.; Rousset, R.; Morchain, J. Effect of tank size on kLa and mixing time in aerated stirred reactors with non-newtonian fluids. Can. J. Chem. Eng. 2011, 89, 1139–1153. [Google Scholar] [CrossRef]
- Rottava, I.; Batesini, G.; Silva, M.F.; Lerin, L.; de Oliveira, D.; Padilha, F.F.; Toniazzo, G.; Mossi, A.; Cansian, R.L.; Di Luccio, M.; et al. Xanthan gum production and rheological behavior using different strains of Xanthomonas sp. Carbohydr. Polym. 2009, 77, 65–71. [Google Scholar] [CrossRef]
- Badino, A.C.; Facciotti, M.C.R.; Schmidell, W. Volumetric oxygen transfer coefficients (kLa) in batch cultivations involving non-Newtonian broths. Biochem. Eng. J. 2001, 8, 111–119. [Google Scholar] [CrossRef]
- Sinha, J.; Tae Bae, J.; Pil Park, J.; Hyun Song, C.; Won Yun, J. Effect of substrate concentration on broth rheology and fungal morphology during exo-biopolymer production by Paecilomyces japonica in a batch bioreactor. Enzym. Microb. Technol. 2001, 29, 392–399. [Google Scholar] [CrossRef]
- Pedersen, A.G.; Bundgaard-Nielsen, M.; Nielsen, J.; Villadsen, J.; Hassager, O. Rheological characterization of media containing Penicillium chrysogenum. Biotechnol. Bioeng. 1993, 41, 162–164. [Google Scholar] [CrossRef]
- Riley, G.L.; Tucker, K.G.; Paul, G.C.; Thomas, C.R. Effect of biomass concentration and mycelial morphology on fermentation broth rheology. Biotechnol. Bioeng. 2000, 68, 160–172. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Rheological studies during submerged citric acid fermentation by Aspergillus niger in stirred fermentor using apple pomace ultrafiltration sludge. Food Bioprocess Technol. 2013, 6, 1240–1250. [Google Scholar] [CrossRef]
- Rodríguez Porcel, E.M.; Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; García Sánchez, J.L.; Chisti, Y. Aspergillus terreus Broth Rheology, Oxygen Transfer, and Lovastatin Production in a Gas-Agitated Slurry Reactor. Ind. Eng. Chem. Res. 2006, 45, 4837–4843. [Google Scholar] [CrossRef]
- Richard, A.; Margaritis, A. Rheology, oxygen transfer, and molecular weight characteristics of poly(glutamic acid) fermentation by Bacillus subtilis. Biotechnol. Bioeng. 2003, 82, 299–305. [Google Scholar] [CrossRef]
- Baroutian, S.; Eshtiaghi, N.; Gapes, D.J. Rheology of a primary and secondary sewage sludge mixture: Dependency on temperature and solid concentration. Bioresour. Technol. 2013, 140, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Bacillus thuringiensis fermentation of hydrolyzed sludge – Rheology and formulation studies. Chemosphere 2007, 67, 674–683. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Sahai, V.; Prévost, D.; Valéro, J.R.; Surampalli, R.Y. Bench-scale fermentation of Trichoderma viride on wastewater sludge: Rheology, lytic enzymes and biocontrol activity. Enzym. Microb. Technol. 2007, 41, 764–771. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Wu, J. Rheological behaviors of an exopolysaccharide from fermentation medium of a Cordyceps sinensis fungus (Cs-HK1). Carbohydr. Polym. 2014, 114, 506–513. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Pais, J.; Costa, N.; Oliveira, C.; Mafra, L.; Hilliou, L.; Oliveira, R.; Reis, M.A.M. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour. Technol. 2009, 100, 859–865. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Carvalheira, M.; Costa, N.; Oliveira, R.; Reis, M.A.M. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2009, 78, 549–556. [Google Scholar] [CrossRef]
- Maalej, H.; Hmidet, N.; Boisset, C.; Bayma, E.; Heyraud, A.; Nasri, M. Rheological and emulsifying properties of a gel-like exopolysaccharide produced by Pseudomonas stutzeri AS22. Food Hydrocoll. 2016, 52, 634–647. [Google Scholar] [CrossRef]
- Maalej, H.; Moalla, D.; Boisset, C.; Bardaa, S.; Ayed, H.B.; Sahnoun, Z.; Rebai, T.; Nasri, M.; Hmidet, N. Rhelogical, dermal wound healing and in vitro antioxidant properties of exopolysaccharide hydrogel from Pseudomonas stutzeri AS22. Colloids Surf. B Biointerfaces 2014, 123, 814–824. [Google Scholar] [CrossRef]
- Goudar, C.T.; Strevett, K.A.; Shah, S.N. Influence of microbial concentration on the rheology of non-Newtonian fermentation broths. Appl. Microbiol. Biotechnol. 1999, 51, 310–315. [Google Scholar] [CrossRef]
- Newton, J.M.; Vlahopoulou, J.; Zhou, Y. Investigating and modelling the effects of cell lysis on the rheological properties of fermentation broths. Biochem. Eng. J. 2017, 121, 38–48. [Google Scholar] [CrossRef]
- Papapostolou, A.; Karasavvas, E.; Chatzidoukas, C. Oxygen mass transfer limitations set the performance boundaries of microbial PHA production processes—A model-based problem investigation supporting scale-up studies. Biochem. Eng. J. 2019, 148, 224–238. [Google Scholar] [CrossRef]
- Arjunwadkar, S.J.; Sarvanan, K.; Kulkarni, P.R.; Pandit, A.B. Gas-liquid mass transfer in dual impeller bioreactor. Biochem. Eng. J. 1998, 1, 99–106. [Google Scholar] [CrossRef]
- Buchholz, H.; Buchholz, R.; Niebeschütz, H.; Schügerl, K. Absorption of oxygen in highly viscous newtonian and non-Newtonian fermentation model media in bubble column bioreactors. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 115–126. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Gómez, E. Mass transfer coefficient in stirred tank reactors for xanthan gum solutions. Biochem. Eng. J. 1998, 1, 1–10. [Google Scholar] [CrossRef]
- Herbst, H.; Schumpe, A.; Deckwer, W.D. Xanthan production in stirred tank fermenters: Oxygen transfer and scale-up. Chem. Eng. Technol. 1992, 15, 425–434. [Google Scholar] [CrossRef]
- Martín, M.; Montes, F.J.; Galán, M.A. Mass transfer rates from bubbles in stirred tanks operating with viscous fluids. Chem. Eng. Sci. 2010, 65, 3814–3824. [Google Scholar] [CrossRef]
- Puthli, M.S.; Rathod, V.K.; Pandit, A.B. Gas–liquid mass transfer studies with triple impeller system on a laboratory scale bioreactor. Biochem. Eng. J. 2005, 23, 25–30. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Z.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of MCL-PHA with elevated 3-hydroxynonanoate content. AMB Express 2013, 3, 50. [Google Scholar] [CrossRef]
- Sharma, P.K.; Fu, J.; Cicek, N.; Sparling, R.; Levin, D.B. Kinetics of medium-chain-length polyhydroxyalkanoate production by a novel isolate of Pseudomonas putida LS46. Can. J. Microbiol. 2012, 58, 982–989. [Google Scholar] [CrossRef]
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.; Levin, D.B.; Cicek, N. Microaerophilic environments improve the productivity of medium chain length polyhydroxyalkanoate biosynthesis from fatty acids in Pseudomonas putida LS46. Process Biochem. 2017, 59, 18–25. [Google Scholar] [CrossRef]
- Ramsay, B.A.; Lomaliza, K.; Chavarie, C.; Dubé, B.; Ramsay, J.A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl. Environ. Microbiol. 1990, 56, 2093–2098. [Google Scholar] [Green Version]
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.; Levin, D.B.; Cicek, N. Carbon flux to growth or polyhydroxyalkanoate synthesis under microaerophilic conditions is affected by fatty acid chain-length in Pseudomonas putida LS46. Appl. Microbiol. Biotechnol. 2018, 102, 6437–6449. [Google Scholar] [CrossRef]
- Blunt, W.; Hossain, M.D.E.; Gapes, D.J.; Sparling, R.; Levin, D.B.; Cicek, N. Real-Time Monitoring of Microbial Fermentation End-Products in Biofuel Production with Titrimetric Off-Gas Analysis (TOGA). Biol. Eng. Trans. 2014, 6, 203–219. [Google Scholar]
- Yang, H.; Wei, F.; Hu, K.; Zhou, G.; Lyu, J. Comparison of rheometric devices for measuring the rheological parameters of debris flow slurry. J. Mt. Sci. 2015, 12, 1125–1134. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Viles, F.J.; Silverman, L. Determination of Starch and Cellulose with Anthrone. Anal. Chem. 1949, 21, 950–953. [Google Scholar] [CrossRef]
- Newton, J.M.; Schofield, D.; Vlahopoulou, J.; Zhou, Y. Detecting cell lysis using viscosity monitoring in E. coli fermentation to prevent product loss. Biotechnol. Prog. 2016, 32, 1069–1076. [Google Scholar] [CrossRef]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2013; ISBN 978-0-12-220851-5. [Google Scholar]
- Wagner, N.J.; Brady, J.F. Shear thickening in colloidal dispersions. Phys. Today 2009, 62, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Jaeger, H.M. Shear thickening in concentrated suspensions: Phenomenology, mechanisms and relations to jamming. Rep. Prog. Phys. 2014, 77, 046602. [Google Scholar] [CrossRef]
- Fernandez, N.; Mani, R.; Rinaldi, D.; Kadau, D.; Mosquet, M.; Lombois-Burger, H.; Cayer-Barrioz, J.; Herrmann, H.J.; Spencer, N.D.; Isa, L. Microscopic Mechanism for Shear Thickening of Non-Brownian Suspensions. Phys. Rev. Lett. 2013, 111, 108301. [Google Scholar] [CrossRef] [Green Version]
- van Egmond, J.W. Shear-thickening in suspensions, associating polymers, worm-like micelles, and poor polymer solutions. Curr. Opin. Colloid Interface Sci. 1998, 3, 385–390. [Google Scholar] [CrossRef]
- Ballard, M.J.; Buscall, R.; Waite, F.A. The theory of shear-thickening polymer solutions. Polymer 1988, 29, 1287–1293. [Google Scholar] [CrossRef]
- Levine, A.C.; Sparano, A.; Twigg, F.F.; Numata, K.; Nomura, C.T. Influence of Cross-Linking on the Physical Properties and Cytotoxicity of Polyhydroxyalkanoate (PHA) Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 567–576. [Google Scholar] [CrossRef]
- Bassas, M.; Diaz, J.; Rodriguez, E.; Espuny, M.J.; Prieto, M.J.; Manresa, A. Microscopic examination in vivo and in vitro of natural and cross-linked polyunsaturated mclPHA. Appl. Microbiol. Biotechnol. 2008, 78, 587–596. [Google Scholar] [CrossRef]
- Hazer, B.; Demirel, S.I.; Borcakli, M.; Eroglu, M.S.; Cakmak, M.; Erman, B. Free radical crosslinking of unsaturated bacterial polyesters obtained from soybean oily acids. Polym. Bull. 2001, 46, 389–394. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A.; Liu, C.K. Glucose/lipid mixed substrates as a means of controlling the properties of medium chain length poly(hydroxyalkanoates). Biomacromolecules 2001, 2, 211–216. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Levery, S.B.; Kim, J.S.; Reuhs, B.L.; Lion, L.W.; Ghiorse, W.C. Structure and carbohydrate analysis of the exopolysaccharide capsule of Pseudomonas putida G7. Environ. Microbiol. 2001, 3, 774–784. [Google Scholar] [CrossRef]
- Celik, G.Y.; Aslim, B.; Beyatli, Y. Characterization and production of the exopolysaccharide (EPS) from Pseudomonas aeruginosa G1 and Pseudomonas putida G12 strains. Carbohydr. Polym. 2008, 73, 178–182. [Google Scholar] [CrossRef]
- Wrangstadh, M.; Conway, P.L.; Kjelleberg, S. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch. Microbiol. 1986, 145, 220–227. [Google Scholar] [CrossRef]
- Chang, W.S.; van de Mortel, M.; Nielsen, L.; Nino de Guzman, G.; Li, X.; Halverson, L.J. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J. Bacteriol. 2007, 189, 8290–8299. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Liu, Y.; Zhang, J.; Ulstrup, J.; Molin, S. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development: EPS-mediated biofilm development. Environ. Microbiol. 2011, 13, 1705–1717. [Google Scholar] [CrossRef]
- Steinberger, R.E.; Holden, P.A. Macromolecular composition of unsaturated Pseudomonas aeruginosa biofilms with time and carbon source. Biofilms 2004, 1, 37–47. [Google Scholar] [CrossRef]
- Banik, R.M.; Kanari, B.; Upadhyay, S.N. Exopolysaccharide of the gellan family: Prospects and potential. World J. Microbiol. Biotechnol. 2000, 16, 407–414. [Google Scholar] [CrossRef]
- Jahn, A.; Griebe, T.; Nielsen, P.H. Composition of Pseudomonas putida biofilms: Accumulation of protein in the biofilm matrix. Biofouling 1999, 14, 49–57. [Google Scholar] [CrossRef]
- Wigneswaran, V.; Nielsen, K.F.; Sternberg, C.; Jensen, P.R.; Folkesson, A.; Jelsbak, L. Biofilm as a production platform for heterologous production of rhamnolipids by the non-pathogenic strain Pseudomonas putida KT2440. Microb. Cell Factories 2016, 15, 181. [Google Scholar] [CrossRef]
- Gutiérrez-Gómez, U.; Servín-González, L.; Soberón-Chávez, G. Role of β-oxidation and de novo fatty acid synthesis in the production of rhamnolipids and polyhydroxyalkanoates by Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 3753–3760. [Google Scholar] [CrossRef]
- Read, R.R.; Costerton, J.W. Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can. J. Microbiol. 1987, 33, 1080–1090. [Google Scholar] [CrossRef]
- Royce, L.A.; Liu, P.; Stebbins, M.J.; Hanson, B.C.; Jarboe, L.R. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl. Microbiol. Biotechnol. 2013, 97, 8317–8327. [Google Scholar] [CrossRef]
- Cerrone, F.; Duane, G.; Casey, E.; Davis, R.; Belton, I.; Kenny, S.T.; Guzik, M.W.; Woods, T.; Babu, R.P.; O’Connor, K. Fed-batch strategies using butyrate for high cell density cultivation of Pseudomonas putida and its use as a biocatalyst. Appl. Microbiol. Biotechnol. 2014, 98, 9217–9228. [Google Scholar] [CrossRef]
- Maclean, H.; Sun, Z.; Ramsay, J.A.; Ramsay, A.B. Decaying exponential feeding of nonanoic acid for the production of medium-chain-length poly(3-hydroxyalkanoates) by Pseudomonas putida KT2440. Can. J. Chem. 2008, 86, 564–569. [Google Scholar] [CrossRef]
- Koller, M.; Sandholzer, D.; Salerno, A.; Braunegg, G.; Narodoslawsky, M. Biopolymer from industrial residues: Life cycle assessment of poly(hydroxyalkanoates) from whey. Resour. Conserv. Recycl. 2013, 73, 64–71. [Google Scholar] [CrossRef]
- Coats, E.R.; Watson, B.S.; Brinkman, C.K. Polyhydroxyalkanoate synthesis by mixed microbial consortia cultured on fermented dairy manure: Effect of aeration on process rates/yields and the associated microbial ecology. Water Res. 2016, 106, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Da Cruz Pradella, J.G.; Taciro, M.K.; Mateus, A.Y.P. High-cell-density poly (3-hydroxybutyrate) production from sucrose using Burkholderia sacchari culture in airlift bioreactor. Bioresour. Technol. 2010, 101, 8355–8360. [Google Scholar] [CrossRef]
- Brown, E.; Forman, N.A.; Orellana, C.S.; Zhang, H.; Maynor, B.W.; Betts, D.E.; DeSimone, J.M.; Jaeger, H.M. Generality of shear thickening in dense suspensions. Nat. Mater. 2010, 9, 220–224. [Google Scholar] [CrossRef] [Green Version]





| Sample | Power Law Constant: Viscosity (mPa·s) | Power Law Constant: Rate Index | Power Law: Regression |
|---|---|---|---|
| Mean ± St. Dev. | Mean ± St. Dev. | Mean ± St. Dev. | |
| 0 h (<0.2 g·L−1) | 0.16 ± 0.02 | 1.46 ± 0.02 | 1.00 ± 0.00 |
| 6 h (2.1 g·L−1) | 0.22 ± 0.01 | 1.41 ± 0.01 | 1.00 ± 0.00 |
| 10 h (8.7 g·L−1) | 0.45 ± 0.06 | 1.32 ± 0.02 | 1.00 ± 0.00 |
| 14 h (16.1 g·L−1) | 0.55 ± 0.15 | 1.35 ± 0.04 | 1.00 ± 0.00 |
| 18 h (22 g·L−1) | 0.62 ± 0.18 | 1.35 ± 0.05 | 1.00 ± 0.00 |
| 22 h (25.3 g·L−1) | 0.80 ± 0.17 | 1.31 ± 0.03 | 0.99 ± 0.00 |
| 12 h supernatant | 0.32 ± 0.02 | 1.37 ± 0.01 | 1.00 ± 0.00 |
| 16 h supernatant | 1.91 ± 0.03 | 1.12 ± 0.00 | 0.99 ± 0.02 |
| 22 h supernatant | 3.15 ± 0.13 | 1.05 ± 0.01 | 0.99 ± 0.00 |
| Sample | Viscosity @ 10 s−1, mPa·s | Shear Thickening Onset, s−1 |
|---|---|---|
| Mean ± St.Dev | Mean ± St.Dev | |
| 0 h (<0.2 g·L−1) | 1.01 ± 0.06 | 76.5 ± 13.0 |
| 6 h (2.1 g·L−1) | 1.01 ± 0.02 | 94.8 ± 0.6 |
| 10 h (8.7 g·L−1) | 1.59 ± 0.01 | 151.1 ± 0.6 |
| 14 h (16.1 g·L−1) | 4.86 ± 0.21 | 258.9 ± 18.1 |
| 18 h (22 g·L−1) | 8.10 ± 0.15 | 294.9 ± 9.1 |
| 22 h (25.3 g·L−1) | 9.22 ± 0.11 | 293.3 ± 12.5 |
| 12 h supernatant | 1.71 ± 0.01 | 156.3 ± 2.2 |
| 16 h supernatant | 4.42 ± 0.07 | 218.9 ± 2.6 |
| 22 h supernatant | 6.81 ± 0.09 | 256.6 ± 8.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blunt, W.; Gaugler, M.; Collet, C.; Sparling, R.; Gapes, D.J.; Levin, D.B.; Cicek, N. Rheological Behavior of High Cell Density Pseudomonas putida LS46 Cultures during Production of Medium Chain Length Polyhydroxyalkanoate (PHA) Polymers. Bioengineering 2019, 6, 93. https://doi.org/10.3390/bioengineering6040093
Blunt W, Gaugler M, Collet C, Sparling R, Gapes DJ, Levin DB, Cicek N. Rheological Behavior of High Cell Density Pseudomonas putida LS46 Cultures during Production of Medium Chain Length Polyhydroxyalkanoate (PHA) Polymers. Bioengineering. 2019; 6(4):93. https://doi.org/10.3390/bioengineering6040093
Chicago/Turabian StyleBlunt, Warren, Marc Gaugler, Christophe Collet, Richard Sparling, Daniel J. Gapes, David B. Levin, and Nazim Cicek. 2019. "Rheological Behavior of High Cell Density Pseudomonas putida LS46 Cultures during Production of Medium Chain Length Polyhydroxyalkanoate (PHA) Polymers" Bioengineering 6, no. 4: 93. https://doi.org/10.3390/bioengineering6040093