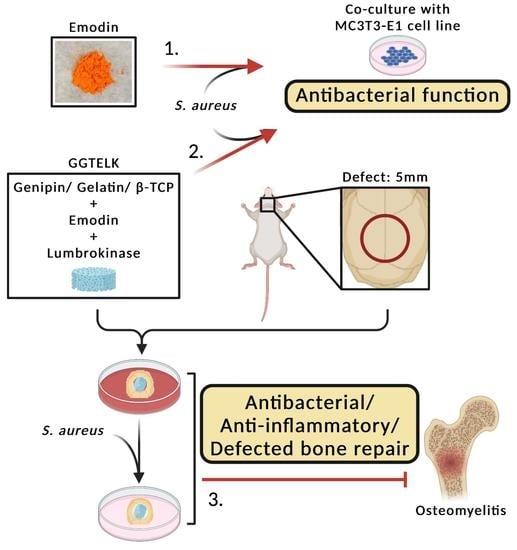
Ex Vivo Model to Evaluate the Antibacterial and Anti-Inflammatory Effects of Gelatin–Tricalcium Phosphate Composite Incorporated with Emodin and Lumbrokinase for Bone Regeneration
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Antibacterial Assay
2.2.1. Turbidimetric Assay
2.2.2. Microtiter Plate Biofilm Formation Assay
2.3. Cell Viability Assay—MTT Assay
2.4. Analysis of ALP (Alkaline phosphatase) Activity
2.5. Analysis of TRAP (Tartrate-Resistant Acid Phosphatase) Assay
2.6. IL 6 ELISA Assay
2.7. Preparation and Characterization of Porous GGTELK Composites
2.7.1. Preparation of Porous GGT and GGTELK Composites
2.7.2. Drug Release Assay
2.7.3. Degradation Assay
2.8. Evaluation of GGTELK Scaffold Using an Ex Vivo Bone Defect Model
2.9. Statistical Analysis
3. Results
3.1. The Antibacterial Effect of Lumbrokinase and Emodin Treatments
3.2. Effects of Emodin and Lubrokinase Treatment on Osteoblast Cells
3.2.1. Lumbrokinase-Activated Osteoblast Differentiation
3.2.2. Lumbrokinase-Increased Osteoblast Mobility
3.3. Effects of Emodin and Lubrokinase Treatment on Osteoclast Cells
3.3.1. LK- and Emodin-Inhibited TRAP Activity Stimulated by RANKL in RAW264.7 Cells
3.3.2. LK and Emodin-Decreased IL-6 Levels in RAW264.7 Cells
3.4. Characterization of GGT and GGTELK Scaffolds
3.4.1. Cumulative Drug-Released Concentration and Degradation Rate of GGT
3.4.2. Biocompatibility of GGTELK
3.4.3. Antibacterial Effect of GGTELK
3.4.4. Antibacterial and Anti-Inflammatory Effects of GGTELK Assessed in an Ex Vivo Bone Defect Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, W.S.; Rayan, F.; Dhinsa, B.S.; Marsh, D. An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: How far are we? Stem Cells Int. 2012, 2012, 236231. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Pirisi, L.; Pennestri, F.; Vigano, M.; Banfi, G. Prevalence and burden of orthopaedic implantable-device infections in Italy: A hospital-based national study. BMC Infect. Dis. 2020, 20, 337. [Google Scholar] [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Gottenbos, B.; Busscher, H.J.; Van Der Mei, H.C.; Nieuwenhuis, P. Pathogenesis and prevention of biomaterial centered infections. J. Mater. Sci. Mater. Med. 2002, 13, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Engineering. C Mater. Biol. Appl. 2021, 118, 111382. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- Bigham, A.; Saudi, A.; Rafienia, M.; Rahmati, S.; Bakhtiyari, H.; Salahshouri, F.; Sattary, M.; Hassanzadeh-Tabrizi, S.A. Electrophoretically deposited mesoporous magnesium silicate with ordered nanopores as an antibiotic-loaded coating on surface-modified titanium. Mater. Sci. Engineering. C Mater. Biol. Appl. 2019, 96, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Albright, V.; Zhuk, I.; Wang, Y.; Selin, V.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C.; Sukhishvili, S.A. Self-defensive antibiotic-loaded layer-by-layer coatings: Imaging of localized bacterial acidification and pH-triggering of antibiotic release. Acta Biomater. 2017, 61, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive antibacterial biomaterial surfaces and their applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Beito, B.; Oliveira, M.; Tudela Martins, M.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for Antimicrobial Peptides Immobilization on Surfaces to Prevent Biofilm Growth on Biomedical Devices. Antibiotics 2021, 11, 13. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Murayama, A.; Ajiki, T.; Hayashi, Y.; Takeshita, K. A unidirectional porous beta-tricalcium phosphate promotes angiogenesis in a vascularized pedicle rat model. J. Orthop. Sci. 2019, 24, 1118–1124. [Google Scholar] [CrossRef]
- Yao, C.H.; Tsai, H.M.; Chen, Y.S.; Liu, B.S. Fabrication and evaluation of a new composite composed of tricalcium phosphate, gelatin, and Chinese medicine as a bone substitute. J. Biomed. Mater. Research. Part B Appl. Biomater. 2005, 75, 277–288. [Google Scholar] [CrossRef]
- Yao, C.H.; Liu, B.S.; Hsu, S.H.; Chen, Y.S. Calvarial bone response to a tricalcium phosphate-genipin crosslinked gelatin composite. Biomaterials 2005, 26, 3065–3074. [Google Scholar] [CrossRef]
- Yan, X.M.; Kim, C.H.; Lee, C.K.; Shin, J.S.; Cho, I.H.; Sohn, U.D. Intestinal Absorption of Fibrinolytic and Proteolytic Lumbrokinase Extracted from Earthworm, Eisenia andrei. Korean J. Physiol. Pharmacol. 2010, 14, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.Y.; Tull, L.; Cooper, E.; Wang, N.; Liu, D. Recombinant protein production of earthworm lumbrokinase for potential antithrombotic application. Evid.-Based Complement. Altern. Med. Ecam 2013, 2013, 783971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanassche, T.; Peetermans, M.; Van Aelst, L.N.; Peetermans, W.E.; Verhaegen, J.; Missiakas, D.M.; Schneewind, O.; Hoylaerts, M.F.; Verhamme, P. The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. J. Infect. Dis. 2013, 208, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; O’Gara, J.P.; O’Neill, E. Novel Treatment of Staphylococcus aureus Device-Related Infections Using Fibrinolytic Agents. Antimicrob. Agents Chemother. 2018, 62, e02008-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapotoczna, M.; McCarthy, H.; Rudkin, J.K.; O’Gara, J.P.; O’Neill, E. An Essential Role for Coagulase in Staphylococcus aureus Biofilm Development Reveals New Therapeutic Possibilities for Device-Related Infections. J. Infect. Dis. 2015, 212, 1883–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Gao, J.; Pang, X.; Chen, A.; Wang, Y. Molecular Mechanisms of Action of Emodin: As an Anti-Cardiovascular Disease Drug. Front. Pharmacol. 2020, 11, 559607. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, X.; Yin, Z.; Jia, R.; Li, Z.; Zhou, X.; Zou, Y.; Li, L.; Yin, L.; Yue, G.; et al. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol. Res. 2016, 186–187, 139–145. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, J.H.; Li, X.; Hwangbo, K.; Hwang, S.L.; Taketomi, Y.; Murakami, M.; Chang, Y.C.; Kim, C.H.; Son, J.K.; et al. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem. Pharmacol. 2011, 82, 1700–1708. [Google Scholar] [CrossRef]
- Alisi, A.; Pastore, A.; Ceccarelli, S.; Panera, N.; Gnani, D.; Bruscalupi, G.; Massimi, M.; Tozzi, G.; Piemonte, F.; Nobili, V. Emodin prevents intrahepatic fat accumulation, inflammation and redox status imbalance during diet-induced hepatosteatosis in rats. Int. J. Mol. Sci. 2012, 13, 2276–2289. [Google Scholar] [CrossRef]
- Chu, X.; Wei, M.; Yang, X.; Cao, Q.; Xie, X.; Guan, M.; Wang, D.; Deng, X. Effects of an anthraquinone derivative from Rheum officinale Baill, emodin, on airway responses in a murine model of asthma. Food Chem. Toxicol. 2012, 50, 2368–2375. [Google Scholar] [CrossRef]
- Fu, Y.T.; Sheu, S.Y.; Chen, Y.S.; Chen, K.Y.; Yao, C.H. Porous gelatin/tricalcium phosphate/genipin composites containing lumbrokinase for bone repair. Bone 2015, 78, 15–22. [Google Scholar] [CrossRef]
- Li, T.; Lu, Y.; Zhang, H.; Wang, L.; Beier, R.C.; Jin, Y.; Wang, W.; Li, H.; Hou, X. Antibacterial Activity and Membrane-Targeting Mechanism of Aloe-Emodin against Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 621866. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cheon, Y.H.; Kwak, S.C.; Baek, J.M.; Yoon, K.H.; Lee, M.S.; Oh, J. Emodin regulates bone remodeling by inhibiting osteoclastogenesis and stimulating osteoblast formation. J. Bone Miner. Res. 2014, 29, 1541–1553. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Wang, X.; Li, J.; Hu, F. Emodin inhibits ATP-induced IL-1beta secretion, ROS production and phagocytosis attenuation in rat peritoneal macrophages via antagonizing P2X(7) receptor. Pharm. Biol. 2014, 52, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, S.A.; Huang, C.H.; Su, H.H.; Chen, Y.H.; Chang, J.T.; Huang, S.S. Sirt1 Activation by Post-ischemic Treatment with Lumbrokinase Protects against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2018, 9, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Log (CFU/mL) | 0 h | 24 h |
|---|---|---|
| Control | 5.6 ± 0.2 | 9.6 ± 0.1 |
| 8 µg/mL Emodin | 5.6 ± 0.2 | 6.4 ± 0.3 |
| 1 µg/mL Lumbrokinase | 5.6 ± 0.2 | 9.5 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-L.; Hsu, Y.-M.; Lin, M.-L.; Chen, S.-S.; Lai, Y.-H.; Huang, C.-H.; Yao, C.-H. Ex Vivo Model to Evaluate the Antibacterial and Anti-Inflammatory Effects of Gelatin–Tricalcium Phosphate Composite Incorporated with Emodin and Lumbrokinase for Bone Regeneration. Bioengineering 2023, 10, 906. https://doi.org/10.3390/bioengineering10080906
Wang W-L, Hsu Y-M, Lin M-L, Chen S-S, Lai Y-H, Huang C-H, Yao C-H. Ex Vivo Model to Evaluate the Antibacterial and Anti-Inflammatory Effects of Gelatin–Tricalcium Phosphate Composite Incorporated with Emodin and Lumbrokinase for Bone Regeneration. Bioengineering. 2023; 10(8):906. https://doi.org/10.3390/bioengineering10080906
Chicago/Turabian StyleWang, Wen-Ling, Yuan-Man Hsu, Meng-Liang Lin, Shih-Shun Chen, Yi-Hui Lai, Chiung-Hua Huang, and Chun-Hsu Yao. 2023. "Ex Vivo Model to Evaluate the Antibacterial and Anti-Inflammatory Effects of Gelatin–Tricalcium Phosphate Composite Incorporated with Emodin and Lumbrokinase for Bone Regeneration" Bioengineering 10, no. 8: 906. https://doi.org/10.3390/bioengineering10080906