Blood Clotting Dissolution in the Presence of a Magnetic Field and Preliminary Study with MG63 Osteoblast-like Cells—Further Developments for Guided Bone Regeneration?
Abstract
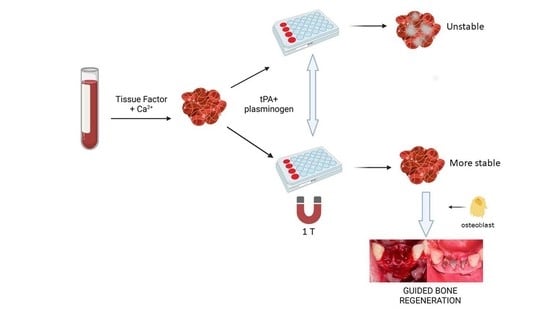
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Whole Blood Clot Preparation
- (i)
- WB clots were formed by mixing 100 μL of whole blood with the same volume of RPMI 1640 and 5 or 10 μL of 10% solution of CaCl2 (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany); the final concentrations obtained with these dilutions were 20 and 40 mM, respectively.
- (ii)
- WB clots were formed by mixing 100 μL of whole blood with 66 μL of RPMI 1640 and 34 μL of a clotting mixture containing RecombiPlasTin2G® (15% v/v) and 67 mM CaCl2 in PBS.
2.3. WB Clot Volume and Weight Determination
2.4. Enzymatic Dissolution of Plasma Clots by Trypsin
2.5. Enzymatic Dissolution of Plasma Clots by Fibrinolytic Agents
2.6. Effect of Magnetic Field on Cell Proliferation in WB Clots
2.7. Statistical Analysis
3. Results
3.1. Clot Formation
3.2. Determination of WB Clot Stability by Spectrophotometric Assay
3.3. Clot Dissolution by Trypsin
3.4. Clot Dissolution by tPA
3.5. Effect of the Magnetic Field on Cell Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, D.; Habibovic, P. Biomineralization-Inspired Material Design for Bone Regeneration. Adv. Healthc. Mater. 2018, 7, e1800700. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and Cellular Aspects of Socket Healing in the Absence and Presence of Graft Materials and Autologous Platelet Concentrates: A Focused Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Murray, G.; Holden, R.; Roschlau, W. Experimental and clinical study of new growth of bone in a cavity. Am. J. Surg. 1957, 93, 385–387. [Google Scholar] [CrossRef]
- Melcher, A.H.; Dreyer, C. Protection of the blood clot in healing circumscribed bone defects. J. Bone Jt. Surg. 1962, 44-B, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Friis, T.; Glatt, V.; Crawford, R.; Xiao, Y. Structural properties of fracture haematoma: Current status and future clinical implications. J. Tissue Eng. Regen. Med. 2017, 11, 2864–2875. [Google Scholar] [CrossRef]
- Lundgren, S.; Andersson, S.; Sennerby, L. Spontaneous bone formation in the maxillary sinus after removal of a cyst: Coincidence or consequence? Clin. Implant Dent. Relat. Res. 2003, 5, 78–81. [Google Scholar] [CrossRef]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral Implants Res. 2003, 14, 63–71. [Google Scholar] [CrossRef]
- Chipaila, N.; Marini, R.; Sfasciotti, G.L.; Cielo, A.; Bonanome, L.; Monaco, A. Graftless sinus augmentation technique with contextual placement of implants: A case report. J. Med. Case Rep. 2014, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Milillo, L.; Cinone, F.; Lo Presti, F.; Lauritano, D.; Petruzzi, M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials 2021, 14, 6642. [Google Scholar] [CrossRef]
- Milillo, L.; Petruzzi, M. Guided Bone Regeneration with Occlusive Titanium Barrier: A Case Report and Clinical Considerations. Biomimetics 2023, 8, 106. [Google Scholar] [CrossRef]
- Lambert, F.; Leonard, A.; Drion, P.; Sourice, S.; Layrolle, P.; Rompen, E. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin. Oral Implants Res. 2011, 22, 538–545. [Google Scholar] [CrossRef]
- Tamura, T.; Fukase, Y.; Goke, E.; Yamada, Y.; Sato, S.; Nishiyama, M.; Ito, K. Three-dimensional evaluation for augmented bone using guided bone regeneration. J. Periodontal. Res. 2005, 40, 269–276. [Google Scholar] [CrossRef]
- Yamada, Y.; Nanba, K.; Ito, K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin. Oral Implants Res. 2003, 14, 455–463. [Google Scholar] [CrossRef]
- Ezirganli, S.; Polat, S.; Baris, E.; Tatar, I.; Celik, H.H. Comparative investigation of the effects of different materials used with a titanium barrier on new bone formation. Clin. Oral Implants Res. 2013, 24, 312–319. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, J.; Long, Y.; Xie, Y.; Nie, J.; Chen, L. Magnetic Materials in Promoting Bone Regeneration. Front. Mater. 2019, 6, 268. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ohsaki, Y.; Goto, T.; Nakasima, A.; Iijima, T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J. Dent. Res. 2003, 82, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Hadjiargyrou, M.; DiMasi, E.; Meng, Y.; Simon, M.; Tan, Z.; Rafailovich, M.H. The role of moderate static magnetic fields on biomineralization of osteoblasts on sulfonated polystyrene films. Biomaterials 2011, 32, 7831–7838. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Leesungbok, R.; Lee, S.W.; Lee, H.W.; Park, S.H.; Mah, S.J.; Ahn, S.J. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics 2015, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.M.; Sinclair, P.M. Effect of pulsed electromagnetic fields on orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, M.A.; Sinclair, P.M.; Kusy, R.P. The effects of samarium-cobalt magnets and pulsed electromagnetic fields on tooth movement. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 578–588. [Google Scholar] [CrossRef]
- Xu, S.; Okano, H.; Tomita, N.; Ikada, Y. Recovery Effects of a 180 mT Static Magnetic Field on Bone Mineral Density of Osteoporotic Lumbar Vertebrae in Ovariectomized Rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 620984. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Wang, Q.; Zhu, T.; Wang, H.; Liu, B.; Wang, Y.; Liu, Z.; Liu, X.; Fan, D.; Wang, X. Recent Advances of Magnetic Nanomaterials in Bone Tissue Repair. Front. Chem. 2020, 8, 745. [Google Scholar] [CrossRef]
- Bhat, V.; Shenoy, K.; Premkumar, P. Magnets in dentistry. Arch. Med. Health Sci. 2013, 1, 73–79. [Google Scholar] [CrossRef]
- Nelwan, L.N.; Nelwan, S.C.; Meizarini, A.; Nowwarote, N. The Role of Static Magnetic Healing Abutment in Osteoblastic Differentiation to Reduce Marginal Crestal Bone Loss. J. Int. Dent. Med. Res. 2022, 15, 896–898. [Google Scholar]
- Cecoro, G.; Bencivenga, D.; Annunziata, M.; Cennamo, N.; Della Ragione, F.; Formisano, A.; Piccirillo, A.; Stampone, E.; Volpe, P.A.; Zeni, L.; et al. Effects of Magnetic Stimulation on Dental Implant Osseointegration: A Scoping Review. Appl. Sci. 2022, 12, 4496. [Google Scholar] [CrossRef]
- Gallusi, G.; Strappa, E.M.; Monterubbianesi, R.; Ferrante, L.; Sampalmieri, F.; Memè, L. Effect of the Magnetic Field Generated by a New NeFeB Cover Screw on Bone Healing around Endosseous Implants: A Case Series Report from Dental Practice. Appl. Sci. 2023, 13, 268. [Google Scholar] [CrossRef]
- Kim, E.C.; Park, J.; Kwon, I.K.; Lee, S.W.; Park, S.J.; Ahn, S.J. Static magnetic fields promote osteoblastic/cementoblastic differentiation in osteoblasts, cementoblasts, and periodontal ligament cells. J. Periodontal Implant Sci. 2017, 47, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Iwasaka, M.; Ueno, S.; Tsuda, H. Dissolution of thrombus under high gradient magnetic fields. IEEE Trans. Magn. 1996, 32, 5130–5132. [Google Scholar] [CrossRef]
- Gorczynska, E.; Wegrzynowicz, R. The effect of magnetic fields on platelets, blood coagulation and fibrinolysis in guinea pigs. Physiol. Chem. Phys. Med. NMR 1983, 15, 459–468. [Google Scholar]
- Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Vinogradov, V.V. Leach-proof magnetic thrombolytic nanoparticles and coatings of enhanced activity. Sci. Rep. 2016, 6, 28119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Kim, H.; Wu, H.; Gao, Y.; Jiang, X. Sonothrombolysis with magnetic microbubbles under a rotational magnetic field. Ultrasonics 2019, 98, 62–71. [Google Scholar] [CrossRef]
- Leszczak, V.; Smith, B.S.; Popat, K.C. Hemocompatibility of polymeric nanostructured surfaces. J. Biomater. Sci. Polym. Ed. 2013, 24, 1529–1548. [Google Scholar] [CrossRef] [Green Version]
- Sabino, R.M.; Kauk, K.; Movafaghi, S.; Kota, A.; Popat, K.C. Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces. Nanomedicine 2019, 21, 102046. [Google Scholar] [CrossRef]
- Bonnard, T.; Law, L.S.; Tennant, Z.; Hagemeyer, C.E. Development and validation of a high throughput whole blood thrombolysis plate assay. Sci. Rep. 2017, 7, 2346. [Google Scholar] [CrossRef] [Green Version]
- van der Vorm, L.N.; Remijn, J.A.; de Laat, B.; Huskens, D. Effects of Plasmin on von Willebrand Factor and Platelets: A Narrative Review. TH Open 2018, 2, e218–e228. [Google Scholar] [CrossRef] [Green Version]
- Lepore, S.; Milillo, L.; Trotta, T.; Castellani, S.; Porro, C.; Panaro, M.A.; Santarelli, A.; Bambini, F.; Lo Muzio, L.; Conese, M.; et al. Adhesion and growth of osteoblast-like cells on laser-engineered porous titanium surface: Expression and localization of N-cadherin and beta-catenin. J. Biol. Regul. Homeost. Agents 2013, 27, 531–541. [Google Scholar] [PubMed]
- Zhou, X.; Holsbeeks, I.; Impens, S.; Sonnaert, M.; Bloemen, V.; Luyten, F.; Schrooten, J. Noninvasive real-time monitoring by alamarBlue((R)) during in vitro culture of three-dimensional tissue-engineered bone constructs. Tissue Eng. Part C Methods 2013, 19, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Medina, T.; Vaquette, C.; Ivanovski, S. Systematic Comparison of the Effect of Four Clinical-Grade Platelet Rich Hemoderivatives on Osteoblast Behaviour. Int. J. Mol. Sci. 2019, 20, 6243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb. Haemost. 2009, 102, 1169–1175. [Google Scholar] [CrossRef]
- Thor, A.; Rasmusson, L.; Wennerberg, A.; Thomsen, P.; Hirsch, J.M.; Nilsson, B.; Hong, J. The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomaterials 2007, 28, 966–974. [Google Scholar] [CrossRef]
- Wang, X.; Friis, T.E.; Masci, P.P.; Crawford, R.W.; Liao, W.; Xiao, Y. Alteration of blood clot structures by interleukin-1 beta in association with bone defects healing. Sci. Rep. 2016, 6, 35645. [Google Scholar] [CrossRef] [Green Version]
- Murayama, M. Orientation of sickled erythrocytes in a magnetic field. Nature 1965, 206, 420–422. [Google Scholar] [CrossRef]
- Higashi, T.; Yamagishi, A.; Takeuchi, T.; Kawaguchi, N.; Sagawa, S.; Onishi, S.; Date, M. Orientation of erythrocytes in a strong static magnetic field. Blood 1993, 82, 1328–1334. [Google Scholar] [CrossRef] [Green Version]
- Terumasa, H.; Akio, Y.; Tetsuya, T.; Muneyuki, D. Effects of static magnetic fields of erythrocyte rheology. Bioelectrochemistry Bioenerg. 1995, 36, 101–108. [Google Scholar] [CrossRef]
- Higashi, T.; Sagawa, S.; Ashida, N.; Takeuchi, T. Orientation of glutaraldehyde-fixed erythrocytes in strong static magnetic fields. Bioelectromagnetics 1996, 17, 335–338. [Google Scholar] [CrossRef]
- Tao, R.; Huang, K. Reducing blood viscosity with magnetic fields. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011, 84, 011905. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Effects of High Magnetic Fields on the Diffusion of Biologically Active Molecules. Cells 2021, 11, 81. [Google Scholar] [CrossRef]
- Mansouri, N.; Al-Sarawi, S.; Losic, D.; Mazumdar, J.; Clark, J.; Gronthos, S.; O’Hare Doig, R. Biodegradable and biocompatible graphene-based scaffolds for functional neural tissue engineering: A strategy approach using dental pulp stem cells and biomaterials. Biotechnol. Bioeng. 2021, 118, 4217–4230. [Google Scholar] [CrossRef]
- Liu, X.; Laurent, C.; Du, Q.; Targa, L.; Cauchois, G.; Chen, Y.; Wang, X.; de Isla, N. Mesenchymal stem cell interacted with PLCL braided scaffold coated with poly-l-lysine/hyaluronic acid for ligament tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 3042–3052. [Google Scholar] [CrossRef]
- Penolazzi, L.; Mazzitelli, S.; Vecchiatini, R.; Torreggiani, E.; Lambertini, E.; Johnson, S.; Badylak, S.F.; Piva, R.; Nastruzzi, C. Human mesenchymal stem cells seeded on extracellular matrix-scaffold: Viability and osteogenic potential. J. Cell. Physiol. 2012, 227, 857–866. [Google Scholar] [CrossRef]
- Gessmann, J.; Seybold, D.; Peter, E.; Schildhauer, T.A.; Koller, M. Plasma clots gelled by different amounts of calcium for stem cell delivery. Langenbecks Arch. Surg. 2013, 398, 161–167. [Google Scholar] [CrossRef]
- Sollazzo, V.; Palmieri, A.; Pezzetti, F.; Massari, L.; Carinci, F. Effects of pulsed electromagnetic fields on human osteoblastlike cells (MG-63): A pilot study. Clin. Orthop. Relat. Res. 2010, 468, 2260–2277. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.L.; Chang, W.J.; Chiu, K.H.; Hsieh, S.C.; Lee, S.Y.; Lin, C.T.; Chen, C.C.; Huang, H.M. Mechanobiology of MG63 osteoblast-like cells adaptation to static magnetic forces. Electromagn. Biol. Med. 2008, 27, 55–64. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioia, S.; Milillo, L.; Hossain, M.N.; Carbone, A.; Petruzzi, M.; Conese, M. Blood Clotting Dissolution in the Presence of a Magnetic Field and Preliminary Study with MG63 Osteoblast-like Cells—Further Developments for Guided Bone Regeneration? Bioengineering 2023, 10, 888. https://doi.org/10.3390/bioengineering10080888
Di Gioia S, Milillo L, Hossain MN, Carbone A, Petruzzi M, Conese M. Blood Clotting Dissolution in the Presence of a Magnetic Field and Preliminary Study with MG63 Osteoblast-like Cells—Further Developments for Guided Bone Regeneration? Bioengineering. 2023; 10(8):888. https://doi.org/10.3390/bioengineering10080888
Chicago/Turabian StyleDi Gioia, Sante, Lucio Milillo, Md Niamat Hossain, Annalucia Carbone, Massimo Petruzzi, and Massimo Conese. 2023. "Blood Clotting Dissolution in the Presence of a Magnetic Field and Preliminary Study with MG63 Osteoblast-like Cells—Further Developments for Guided Bone Regeneration?" Bioengineering 10, no. 8: 888. https://doi.org/10.3390/bioengineering10080888