The Use of Tactile Sensors in Oral and Maxillofacial Surgery: An Overview
Abstract
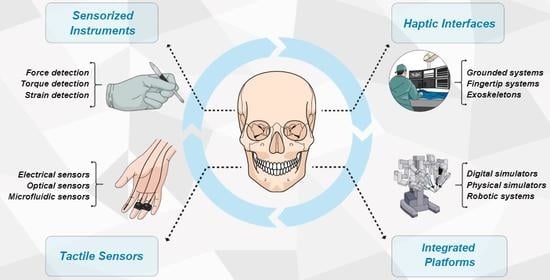
:1. Introduction
- Tactile sensors for instrumented tools;
- Sensorized tools for contact forces detection;
- Haptic interfaces and main actuation principles for remote tactile feedback;
- Integrated platforms for surgery and telemedicine.
2. Materials and Methods
3. Results
3.1. Tactile Sensors for Instrumented Tools: Dentistry and Maxillofacial Applications
3.2. Sensorized Tools for Contact Forces Detection: Dentistry and Maxillofacial Applications
3.3. Main Actuation Principles for Haptic Feedback: Dentistry and Maxillofacial Applications
3.4. Integrated Platforms for Surgery and Telemedicine: Dentistry and Maxillofacial Applications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wang, S.; Zuo, S. A hand-held device with 3-DOF haptic feedback mechanism for microsurgery. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2019, 15, e2025. [Google Scholar] [CrossRef]
- Koehn, J.K.; Kuchenbecker, K.J. Surgeons and non-surgeons prefer haptic feedback of instrument vibrations during robotic surgery. Surg. Endosc. 2015, 29, 2970–2983. [Google Scholar] [CrossRef]
- Liu, H.H.; Li, L.J.; Shi, B.; Xu, C.W.; Luo, E. Robotic surgical systems in maxillofacial surgery: A review. Int. J. Oral Sci. 2017, 9, 63–73. [Google Scholar] [CrossRef]
- Friedrich, D.T.; Scheithauer, M.O.; Greve, J.; Hoffmann, T.K.; Schuler, P.J. Recent advances in robot-assisted head and neck surgery. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2017, 13, e1744. [Google Scholar] [CrossRef]
- Quek, Z.F.; Provancher, W.R.; Okamura, A.M. Evaluation of Skin Deformation Tactile Feedback for Teleoperated Surgical Tasks. IEEE Trans. Haptics 2019, 12, 102–113. [Google Scholar] [CrossRef]
- Chi, C.; Sun, X.; Xue, N.; Li, T.; Liu, C. Recent Progress in Technologies for Tactile Sensors. Sensors 2018, 18, 948. [Google Scholar] [CrossRef] [Green Version]
- Okamura, A.M. Haptic feedback in robot-assisted minimally invasive surgery. Curr. Opin. Urol. 2009, 19, 102–107. [Google Scholar] [CrossRef]
- Zhou, M.; Tse, S.; Derevianko, A.; Jones, D.B.; Schwaitzberg, S.D.; Cao, C.G. Effect of haptic feedback in laparoscopic surgery skill acquisition. Surg. Endosc. 2012, 26, 1128–1134. [Google Scholar] [CrossRef] [Green Version]
- Hagen, M.E.; Meehan, J.J.; Inan, I.; Morel, P. Visual clues act as a substitute for haptic feedback in robotic surgery. Surg. Endosc. 2008, 22, 1505–1508. [Google Scholar] [CrossRef]
- Meccariello, G.; Faedi, F.; AlGhamdi, S.; Montevecchi, F.; Firinu, E.; Zanotti, C.; Cavaliere, D.; Gunelli, R.; Taurchini, M.; Amadori, A.; et al. An experimental study about haptic feedback in robotic surgery: May visual feedback substitute tactile feedback? J. Robot. Surg. 2016, 10, 57–61. [Google Scholar] [CrossRef]
- Iordachita, I.; Sun, Z.; Balicki, M.; Kang, J.U.; Phee, S.J.; Handa, J.; Gehlbach, P.; Taylor, R. A sub-millimetric, 0.25 mN resolution fully integrated fiber-optic force-sensing tool for retinal microsurgery. Int. J. Comput. Assist. Radiol. Surg. 2009, 4, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Bell, B.; Stankowski, S.; Moser, B.; Oliva, V.; Stieger, C.; Nolte, L.P.; Caversaccio, M.; Weber, S. Integrating optical fiber force sensors into microforceps for ORL microsurgery. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1848–1851. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, K.; Xiao, N.; Guo, S. A force acquisition method in a catheter navigation system. In Proceedings of the ICME International Conference on Complex Medical Engineering, Beijing, China, 25–28 May 2013; pp. 633–637. [Google Scholar]
- Park, Y.L.; Elayaperumal, S.; Daniel, B.; Ryu, S.C.; Shin, M.; Savall, J.; Black, R.J.; Moslehi, B.; Cutkosky, M.R. Real-Time Estimation of 3-D Needle Shape and Deflection for MRI-Guided Interventions. IEEE/ASME Trans. Mechatron. 2010, 15, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Frosolini, A.; Franz, L.; Daloiso, A.; de Filippis, C.; Marioni, G. Sudden Sensorineural Hearing Loss in the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Diagnostics 2012, 12, 3139. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ Clin. Res. Ed. 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Tactile sensors for minimally invasive surgery: A review of the state-of-the-art, applications, and perspectives. IEEE Access 2020, 8, 7682–7708. [Google Scholar] [CrossRef]
- Dahiya, R.S.; Metta, G.; Valle, M.; Sandini, G. Tactile sensing-from humans to humanoids. IEEE Trans. Robot. 2010, 26, 1–20. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Oddo, C.M.; Zollo, L.; Silvestri, S.; Guglielmelli, E. Microfabricated tactile sensors for biomedical applications: A review. Biosensors 2014, 4, 422–448. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Zou, K.; Long, J.; Jin, J.; Zhang, D.; Coppola, G.; Sun, W.; Wang, Y.; Ge, Y. Multi-Component FBG-Based Force Sensing Systems by Comparison with Other Sensing Technologies: A Review. IEEE Sens. J. 2018, 18, 7345–7357. [Google Scholar] [CrossRef]
- Lo Presti, D.; Massaroni, C.; Jorge Leitao, C.S.; De Fatima Domingues, M.; Sypabekova, M.; Barrera, D.; Floris, I.; Massari, L.; Maria Oddo, C.; Sales, S.; et al. Fiber bragg gratings for medical applications and future challenges: A review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Liu, T.; Duan, X.; Huang, Q.; Zhao, H.; Guo, Q. Control system for maxillofacial surgery robot: Master-slave, motion control and safety design. In Proceedings of the 2012 ICME International Conference on Complex Medical Engineering (CME), Kobe, Japan, 1–4 July 2012; pp. 203–208. [Google Scholar]
- Nicot, R.; Couly, G.; Ferri, J.; Levaillant, J.M. Three-dimensional printed haptic model from a prenatal surface-rendered oropalatal sonographic view: A new tool in the surgical planning of cleft lip/palate. Int. J. Oral Maxillofac. Surg. 2018, 47, 44–47. [Google Scholar] [CrossRef]
- Mencattelli, M.; Donati, E.; Cultrone, M.; Stefanini, C. Novel universal system for 3-dimensional orthodontic force-moment measurements and its clinical use. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 174–183. [Google Scholar] [CrossRef]
- Pacchierotti, C.; Sinclair, S.; Solazzi, M.; Frisoli, A.; Hayward, V.; Prattichizzo, D. Wearable Haptic Systems for the Fingertip and the Hand: Taxonomy, Review, and Perspectives. IEEE Trans. Haptics 2017, 10, 580–600. [Google Scholar] [CrossRef] [Green Version]
- Pacchierotti, C.; Meli, L.; Chinello, F.; Malvezzi, M.; Prattichizzo, D. Cutaneous haptic feedback to ensure the stability of robotic teleoperation systems. Int. J. Robot. Res. 2015, 34, 1773–1787. [Google Scholar] [CrossRef]
- Prattichizzo, D.; Chinello, F.; Pacchierotti, C.; Malvezzi, M. Towards wearability in fingertip haptics: A 3-DoF wearable device for cutaneous force feedback. IEEE Trans. Haptics 2013, 6, 506–516. [Google Scholar] [CrossRef]
- Frediani, G.; Mazzei, D.; De Rossi, D.E.; Carpi, F. Wearable wireless tactile display for virtual interactions with soft bodies. Front. Bioeng. Biotechnol. 2014, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Sun, P.; Liao, D. A patient-specific haptic drilling simulator based on virtual reality for dental implant surgery. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1861–1870. [Google Scholar] [CrossRef]
- Zheng, F.; Lu, W.F.; Wong, Y.S.; Foong, K.W. An analytical drilling force model and GPU-accelerated haptics-based simulation framework of the pilot drilling procedure for micro-implants surgery training. Comput. Methods Programs Biomed. 2012, 108, 1170–1184. [Google Scholar] [CrossRef]
- Arikatla, V.S.; Tyagi, M.; Enquobahrie, A.; Nguyen, T.; Blakey, G.H.; White, R.; Paniagua, B. High Fidelity Virtual Reality Orthognathic Surgery Simulator. Proc. SPIE Int. Soc. Opt. Eng. 2018, 10576, 1057612. [Google Scholar] [CrossRef]
- Wu, F.; Chen, X.; Lin, Y.; Wang, C.; Wang, X.; Shen, G.; Qin, J.; Heng, P.A. A virtual training system for maxillofacial surgery using advanced haptic feedback and immersive workbench. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2014, 10, 78–87. [Google Scholar] [CrossRef]
- Jing, Z.; Qian, J.; Zhang, H.; He, L.; Li, B.; Qin, J.; Dai, H.; Wei, T.; Weidong, T. Maxillofacial surgical simulation system with haptic feedback. J. Ind. Manag. Optim. 2020, 17, 3645–3657. [Google Scholar]
- Zhang, J.; Li, D.; Liu, Q.; He, L.; Huang, Y.; Li, P. Virtual surgical system in reduction of maxillary fracture. In Proceedings of the IEEE International Conference on Digital Signal Processing (DSP), Singapore, 21–24 July 2015; pp. 1102–1105. [Google Scholar]
- Girod, S.; Schvartzman, S.C.; Gaudilliere, D.; Salisbury, K.; Silva, R. Haptic feedback improves surgeons’ user experience and fracture reduction in facial trauma simulation. J. Rehabil. Res. Dev. 2016, 53, 561–570. [Google Scholar] [CrossRef]
- Nilsson, J.; Nysjö, F.; Nyström, I.; Kämpe, J.; Thor, A. Evaluation of in-house, haptic assisted surgical planning for virtual reduction of complex mandibular fractures. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1059–1068. [Google Scholar] [CrossRef]
- Olsson, P.; Nysjö, F.; Hirsch, J.M.; Carlbom, I.B. A haptics-assisted cranio-maxillofacial surgery planning system for restoring skeletal anatomy in complex trauma cases. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 887–894. [Google Scholar] [CrossRef]
- Medellín-Castillo, H.I.; Govea-Valladares, E.H.; Pérez-Guerrero, C.N.; Gil-Valladares, J.; Lim, T.; Ritchie, J.M. The evaluation of a novel haptic-enabled virtual reality approach for computer-aided cephalometry. Comput. Methods Programs Biomed. 2016, 130, 46–53. [Google Scholar] [CrossRef]
- Zaragoza-Siqueiros, J.; Medellin-Castillo, H.I.; de la Garza-Camargo, H.; Lim, T.; Ritchie, J.M. An integrated haptic-enabled virtual reality system for orthognathic surgery planning. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 499–517. [Google Scholar] [CrossRef]
- Bugdadi, A.; Sawaya, R.; Bajunaid, K.; Olwi, D.; Winkler-Schwartz, A.; Ledwos, N.; Marwa, I.; Alsideiri, G.; Sabbagh, A.J.; Alotaibi, F.E.; et al. Is Virtual Reality Surgical Performance Influenced by Force Feedback Device Utilized? J. Surg. Educ. 2019, 76, 262–273. [Google Scholar] [CrossRef]
- Maliha, S.G.; Diaz-Siso, J.R.; Plana, N.M.; Torroni, A.; Flores, R.L. Haptic, Physical, and Web-Based Simulators: Are They Underused in Maxillofacial Surgery Training? J. Oral Maxillofac. Surg. 2018, 76, 2424.e1–2424.e11. [Google Scholar] [CrossRef]
- Giri, G.S.; Maddahi, Y.; Zareinia, K. An Application-Based Review of Haptics Technology. Robotics 2021, 10, 29. [Google Scholar] [CrossRef]
- Hoshyarmanesh, H.; Zareinia, K.; Lama, S.; Sutherland, G.R. Structural design of a microsurgery-specific haptic device: NeuroArmPLUSHD prototype. Mechatronics 2021, 73, 102481. [Google Scholar] [CrossRef]
- Pacchierotti, C.; Prattichizzo, D.; Kuchenbecker, K.J. Cutaneous Feedback of Fingertip Deformation and Vibration for Palpation in Robotic Surgery. IEEE Trans. Bio-Med. Eng. 2016, 63, 278–287. [Google Scholar] [CrossRef]
- Shujaat, S.; da Costa Senior, O.; Shaheen, E.; Politis, C.; Jacobs, R. Visual and haptic perceptibility of 3D printed skeletal models in orthognathic surgery. J. Dent. 2021, 109, 103660. [Google Scholar] [CrossRef]
- Syed, A.A.; Duan, X.; Kong, X.; Li, M.; Wang, Y.; Huang, Q. Maxillofacial surgical robotic manipulator controlled by haptic device with force feedback. In Proceedings of the 2013 ICME International Conference on Complex Medical Engineering, Beijing, China, 25–28 May 2013; pp. 363–368. [Google Scholar]
- Kim, D.H.; Kim, H.M.; Park, J.S.; Kim, S.W. Virtual Reality Haptic Simulator for Endoscopic Sinus and Skull Base Surgeries. J. Craniofacial Surg. 2020, 31, 1811–1814. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- McArthur, A.; Klugarova, J.; Yan, H.; Florescu, S. Chapter 4: Systematic reviews of text and opinion. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Li, X.; Guo, S.; Shi, P.; Jin, X.; Kawanishi, M. An Endovascular Catheterization Robotic System Using Collaborative Operation with Magnetically Controlled Haptic Force Feedback. Micromachines 2022, 13, 505. [Google Scholar] [CrossRef]
- Alaker, M.; Wynn, G.R.; Arulampalam, T. Virtual reality training in laparoscopic surgery: A systematic review & meta-analysis. Int. J. Surg. 2016, 29, 85–94. [Google Scholar] [CrossRef]
- Postema, R.R.; van Gastel, L.A.; Hardon, S.F.; Bonjer, H.J.; Horeman, T. Haptic exploration improves performance of a laparoscopic training task. Surg. Endosc. 2021, 35, 4175–4182. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Q.; Zhao, M.; Chen, C.; Pan, S.; Yuan, M. Tactile Perception Technologies and Their Applications in Minimally Invasive Surgery: A Review. Front. Physiol. 2020, 11, 611596. [Google Scholar] [CrossRef]
- Ghamraoui, A.K.; Ricotta, J.J. Current and Future Perspectives in Robotic Endovascular Surgery. Curr. Surg. Rep. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Othman, W.; Lai, Z.A.; Abril, C.; Barajas-Gamboa, J.S.; Corcelles, R.; Kroh, M.; Qasaimeh, M.A. Tactile Sensing for Minimally Invasive Surgery: Conventional Methods and Potential Emerging Tactile Technologies. Front. Robot. AI 2022, 8, 705662. [Google Scholar] [CrossRef]
- Pollard, M.; Maugi, R.; Platt, M. Multi-resistive pulse sensor microfluidic device. Analyst 2022, 147, 1417–1424. [Google Scholar] [CrossRef]
- Jing, Q.; Pace, A.; Ives, L.; Husmann, A.; Ćatić, N.; Khanduja, V.; Cama, J.; Kar-Narayan, S. Aerosol-jet-printed, conformable microfluidic force sensors. Cell Rep. Phys. Sci. 2021, 2, 100386. [Google Scholar] [CrossRef]
- Aboh, I.V.; Chisci, G.; Cascino, F.; Parigi, S.; Gennaro, P.; Gabriele, G.; Iannetti, G. Giant palatal schwannoma. J. Craniofac. Surg. 2014, 25, e418-20. [Google Scholar] [CrossRef]
- Cascone, P.; Gennaro, P.; Gabriele, G.; Chisci, G.; Mitro, V.; De Caris, F.; Iannetti, G. Temporomandibular synovial chondromatosis with numerous nodules. J. Craniofac. Surg. 2014, 25, 1114–1115. [Google Scholar] [CrossRef]
- Gennaro, P.; Giovannoni, M.E.; Pini, N.; Aboh, I.V.; Gabriele, G.; Iannetti, G.; Cascino, F. Relationship Between the Quantity of Nerve Exposure During Bilateral Sagittal Split Osteotomy Surgery and Sensitive Recovery. J. Craniofac. Surg. 2017, 28, 1375–1379. [Google Scholar] [CrossRef]

| First Author, Year [Reference] | Country | Type and Grade # | Field | Subjects | Category | Original Technology | Conclusions |
|---|---|---|---|---|---|---|---|
| Arikatla 2018 [31] | USA/India | CR (4/7) | Orthognatic surgery | 1 | Integrated platforms | NA | In the future, we will employ the methods proposed in this paper to build a prototype that simulates the full procedure of the BSSO. |
| Bandari 2016 [17] | Canada | Review (10/12) | MIS and RMIS | NA | TS-IT | NA | There is a relatively small number of recent developments of hybrid sensors for MIS and RMIS. |
| Bugdadi 2018 [40] | Canada | Exp (6/6) | Neurosurgery | 6 * | Haptic feedback; Integrated platforms | NA | To maximize realism of the training experience, educators employing virtual reality simulators may find it useful to assess expert opinion before choosing a force feedback device. |
| Chen 2018 [29] | China | Exp (6/6) | Dentistry | 30 ** | Haptic feedback; Integrated platforms | DISS | The DISS may provide an alternative training method for the surgeons to enhance their dental implant surgical skills and experiences. |
| Dahiya 2010 [18] | Italy | Review (7/12) | Translational | NA | TS-IT | NA | Much work needs to be conducted at the system level before artificial touch can be used in a real-world environment. |
| Frediani 2014 [28] | UK/Italy | Exp (5/6) | Translational | NR | Haptic feedback | bubble like HC-DEA | A novel tactile display able to simulate contact with virtual soft bodies via soft interfaces, offering low weight, no acoustic noise, no heating, scalability, and low power consumption. |
| Giri 2021 [42] | Canada | Review (5/12) | Translational | NA | Haptic feedback | NA | The medicinal world is still skeptical about the usage of haptic devices in surgeries and training. |
| Girod 2015 [35] | USA | Exp (5/6) | Maxillofacial | 10 ** | Haptic feedback; Integrated platforms | Geomagic Touch, 3D Systems | Our advanced haptic surgical planning system enabled surgeons to simulate mandibular fracture repair more accurately and with a better user experience than a CAD system. |
| Hoshyarmanesh 2021 [43] | Canada | Exp (5/6) | MIS and RMIS | NR | Haptic feedback; Integrated platforms | neuroArmPLUS | This manuscript describes the engineering principles behind the design and development of a microsurgery-specific haptic interface. Establishing its clinical use is outside the scope of this work. |
| Kim 2020 [47] | Korea | Exp (4/6) | FESS | NR | Haptic feedback; Integrated platforms | VR haptic platform | VR haptic simulators can improve the skill and confidence of surgical trainees by allowing them to accrue experience in various tasks under different conditions. |
| Liang 2018 [20] | China/Canada | Review (5/12) | Translational | NA | TS-IT | NA | Significant improvements have been achieved in addressing numerous aspects of designing and developing multicomponent opto-electric force sensing systems. |
| Liu 2012 [22] | China | Exp (4/6) | Maxillofacial | NA | ST-CFD | Control System for CMF Robot | The repeatability accuracy experiment showed that the movement of the robot is smooth, stable, and safe. |
| Lo presti 2020 [21] | Spain/Portugal/Italy/Usa | Review (6/12) | Translational | NA | TS-IT | NA | The considerable amount of attention given to FBGs in scientific papers and the growing market interest regarding their applications in medicine underline the strong interest in fulfilling the gap between research and clinical practice. |
| Maliha 2018 [41] | USA | Review (10/12) | Maxillofacial | NA | Haptic feedback | NA | Although seemingly beneficial to the trainee in maxillofacial surgery, simulation in education in this field is an underused commodity because of the significant lack of scientific and validated study designs reported on in the literature thus far. |
| Medellìn-Castillo 2016 [38] | Mexico/UK | CS (4/10) | Maxillofacial | 5 | Haptic feedback | NA | A haptic-enabled approach is feasible in 2D, 21⁄2D, and 3D environments and benefits were obtained in the reduction of measurement errors, lower variability, and reduced task completion times. |
| Mencattelli 2014 [24] | Italy | CR (4/8) | Dentistry | 2 | ST-CFD | Measuring platform | This measuring system allows measurements of 6 orthodontic forces exerted by any orthodontic device. |
| Nicot 2017 [23] | France | CR (4/7) | Maxillofacial | 1 | Haptic feedback | NA | The description of defects through haptic 3D printed models may be the next step in the provision of parental information. |
| Nilsson 2020 [36] | Denmark/Sweden | CS (3/10) | Maxillofacial | 12 | Haptic feedback | NA | In this study, we present an in-house haptic-assisted planning tool with high usability that can be used for preoperative planning and evaluation of complex mandible fractures. |
| Olsson 2013 [37] | Sweden | CR (5/6) | Maxillofacial | 1 | Haptic feedback | NA | Preliminary testing with one surgeon indicates that our haptic planning system has the potential to become a powerful tool that with little training allows a surgeon to complete a complex CMF surgery plan in a short amount of time. |
| Pacchierotti 2015a [26] | Italy | Exp (5/6) | Translational | 15 *** | Haptic feedback | 3-DoF cutaneous haptic device | Cutaneous feedback showed better performance than employing no force feedback at all, but, as expected, it was outperformed by full haptic feedback provided by grounded haptic interfaces. |
| Pacchierotti 2015b [44] | Italy/USA | Exp (5/6) | Translational | 18 *** | Haptic feedback | SynTouch BioTac | Subjects who used a dragging strategy achieved even better results with cutaneous feedback of fingertip vibrations. Subjects also highly preferred conditions providing cutaneous feedback over the one without any haptic feedback. |
| Pacchierotti 2017 [25] | France/Italy | Review (6/12) | Translational | NA | Haptic feedback | NA | The “wearables” technology trend will continue to play a strong role in pushing haptics forward in the coming decade. |
| Prattichizzo 2013 [27] | Italy | Exp (5/6) | Translational | 14 *** | Haptic feedback | Novel 3-DoF wearable display | In comparison to similar existing cutaneous devices, this one has three actuated degrees of freedom and it is able to simulate a contact force with general direction at the fingertip. |
| Saccomandi 2014 [19] | Italy | Review (7/12) | Translational | NA | TS-IT | NA | The growing and continuous research in the field of tactile sensing for biomedical applications will go towards the fusion of many technologies, aiming to enhance the pros of each technique. |
| Shujaat 2021 [45] | Belgium/Sweden | CR (4/7) | Orthognatic surgery | 1 | Haptic feedback | NA | Our findings provide evidence on the anatomical and haptic quality 3D models with various printers which may guide physicians and trainees to select a certain printer and material depending on the task at hand. |
| Syed 2013 [46] | China | Exp (4/6) | Maxillofacial | NR | Haptic feedback | 6-DOF | The repeatability Exp results show that the movement of the manipulator under satisfactory boundaries, which is suitable and fulfills the needs of the surgery. |
| Wu 2014 [32] | China | Exp (4/6) | Maxillofacial | 25 ** | Haptic feedback | VR-MFS | The VR-MFS provides an efficient and cost-effective way to train maxillofacial novices. |
| Zaragoza-Siqueiros 2019 [39] | Mexico/UK | CR (4/7) | Orthognatic surgery | 6 ** | Haptic feedback; Integrated platforms | OSSys | The proposed system integrates the four main stages of the traditional OGS planning process: clinical facial analysis, cephalometric analysis, model surgery, and surgical template generation. |
| Zhang 2015 [34] | China | Exp (4/6) | Maxillofacial | NR | Haptic feedback | Digital VR simulator | This system provides training to medical students, and can also be used in preoperative planning. |
| Zhang 2021 [33] | China | Exp (5/6) | Maxillofacial | 10 ** | Haptic feedback | There are still a lot of technical challenges in the development of virtual surgery. | |
| Zheng 2012 [30] | China | Exp (4/6) | Dentistry | NR | Haptic feedback | This paper presents an effective framework to simulate the pilot-drilling procedure of the micro-implants surgery. |
| Olsson | Nicot | Shujaat | Arikatla | Zaragoza | Mencattelli | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | |
| Were the patient’s demographic characteristics clearly described? | 1 | □ | □ | □ | 1 | 1 | 1 | 1 | 0 | 1 | ||||||||||||||
| Was the patient’s history clearly described and presented as a timeline? | 0 | □ | □ | □ | 0 | 0 | 0 | 0 | 0 | 1 | ||||||||||||||
| Was the current clinical condition of the patient on presentation clearly described? | 1 | □ | □ | □ | 1 | 0 | 0 | 0 | 1 | |||||||||||||||
| Were diagnostic tests or assessment methods and the results clearly described? | □ | □ | □ | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| Was the intervention(s) or treatment procedure(s) clearly described? | 1 | □ | □ | □ | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| Was the post-intervention clinical condition clearly described? | 1 | □ | □ | □ | 0 | 0 | 0 | 0 | 0 | 1 | ||||||||||||||
| Were adverse events (harms) or unanticipated events identified and described? | □ | □ | □ | 1 | 0 | 1 | 1 | 1 | 1 | |||||||||||||||
| Does the case report provide takeaway lessons? | 1 | □ | □ | □ | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| Total | 5/6 | 4/7 | 4/7 | 4/7 | 4/7 | 4/8 |
| Nilsson | Medellìn-Castillo | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Unclear | Not Applicable | Yes | No | Unclear | Not Applicable | |
| Were there clear criteria for inclusion in the case series? | □ | □ | 1 | □ | 1 | |||
| Was the condition measured in a standard, reliable way for all participants included in the case series? | □ | □ | 1 | □ | 1 | |||
| Were valid methods used for identification of the condition for all participants included in the case series? | 1 | □ | □ | □ | 1 | |||
| Did the case series have consecutive inclusion of participants? | 1 | □ | □ | □ | 1 | |||
| Did the case series have complete inclusion of participants? | □ | □ | 1 | □ | 1 | |||
| Was there clear reporting of the demographics of the participants in the study? | □ | 1 | □ | □ | 1 | |||
| Was there clear reporting of clinical information of the participants? | □ | 1 | □ | □ | 1 | |||
| Were the outcomes or follow-up results of cases clearly reported? | □ | 1 | □ | □ | 1 | |||
| Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | □ | 1 | □ | □ | 1 | |||
| Was statistical analysis appropriate? | 1 | □ | □ | □ | 1 | |||
| 3/10 | 4/10 |
| Bugdadi | Chen | Frediani | Girod | Hoshyarmanesh | Kim | Liu | Pacchierotti 2015a | Pacchierotti 2015b | Prattichizzo 2013 | Syed | Wu | Zhang 2015 | Zhang 2021 | Zheng 2012 | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | Yes | No | Unclear | Not applicable | |
| Is the source of the opinion clearly identified? £ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ |
| Does the source of opinion have standing in the field of expertise? § | 1 | □ | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | 1 | □ | □ | □ | □ | □ | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ |
| Are the interests of the relevant population the central focus of the opinion? | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ |
| Is the stated position the result of an analytical process, and is there logic in the opinion expressed? $ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ |
| Is there reference to the extant literature? * | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | 1 | □ | □ | □ | □ | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ |
| Is any incongruence with the literature/sources logically defended? | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ | □ | □ | 1 | □ | □ | □ | 1 | □ | □ |
| Total | 6/6 | 6/6 | 5/6 | 5/6 | 5/6 | 4/6 | 4/6 | 5/6 | 5/6 | 5/6 | 4/6 | 6/6 | 4/6 | 5/6 | 4/6 |
| Bandari | Dahiya | Giri | Liang | Lo presti | Maliha | Pacchierotti | Saccomandi | |
|---|---|---|---|---|---|---|---|---|
| (1) Justification of the article’s importance for the readership. The importance is not justified. 0 The importance is alluded to, but not explicitly justified. The importance is explicitly justified. | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 |
| (2) Statement of concrete aims or formulation of questions. No aims or questions are formulated. 0 Aims are formulated generally but not concretely or in terms of clear questions. One or more concrete aims or questions are formulated. | 2 | 1 | 1 | 0 | 1 | 2 | 0 | 1 |
| (3) Description of the literature search. The search strategy is not presented. The literature search is described briefly. The literature search is described in detail, including search terms and inclusion criteria | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| (4) Referencing Key statements are not supported by references. 0 The referencing of key statements is inconsistent. 1 Key statements are supported by references. 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| (5) Scientific reasoning (e.g., incorporation of appropriate evidence, such as RCTs in clinical medicine). The article’s point is not based on appropriate arguments. 0 Appropriate evidence is introduced selectively. 1 Appropriate evidence is generally present. 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| (6) Appropriate presentation of data (e.g., absolute vs. relative risk; effect sizes without confidence intervals). Data are presented inadequately. 0 Data are often not presented in the most appropriate way. 1 Relevant outcome data are generally presented appropriately. 2 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 2 |
| Total | 10/12 | 7/12 | 5/12 | 5/12 | 6/12 | 10/12 | 6/12 | 7/12 |
| Sensing Principle | Advantages | Disavantages | |
|---|---|---|---|
| Piezoresistors | Resistance variation | Excellent spatial resolution High sensivity Low cost | Low frequency response Low repeatability |
| Capacitive sensors | Capacitance variation due to a mechanical force or moment | Temperature independent High spatial resolution High sensivity | Crosstalk between elements Susceptible to noise Stray capacitance hysteresis |
| Inductive sensors | Magnetic coupling variation due to a mechanical force or moment | High dynamic range High sensivity Linear output | Low frequency response Poor reliability |
| Strain gauges sensors | Change in resistance because of shape deformation | High spatial resolution Easy design Low cost | Largge hysteresis Non-linear response |
| Piezoelectric sensors | Strain polarization | High accuracy High dynamic range High frequency response | Low spatial resolution High temperature susceptibility |
| Optical sensors | Change in wavelenght | High spatial resolution and sensivity Electomagnetically inert | High temperature susceptibility High cost Size limitations |
| First Author, Year [Reference] | Medical Procedure | Type of Simulator | Haptic Device | Simulation’s Software | |
|---|---|---|---|---|---|
| Dentistry | Chen 2018 [29] | DISS | Digital | Omega.6 | CHAI3D 1.1 |
| Zheng 2012 [30] | Micro-implants surgery | Digital | Phantom Desktop | - | |
| Maxillofacial Surgery | Arikatla 2018 [31] | BSSO | Digital | Geomagic Touch | toolkit-iMSTK |
| Wu 2014 [32] | Le-Fort I osteotomy | Digital | Omega.6 | VS2008 CHAI3D | |
| Zhang 2015 [34] | Reduction of maxillary fractures | Digital | Geomagic Phantom | CHAI3D | |
| Zhang 2021 [33] | Open reduction and plate fixation | Digital | Geomagic Phantom desktop X | OpenGL | |
| Kim 2020 [47] | Endoscopic sinus and skull base surgeries | Physical | Geomagic Touch X | Unity3D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navalesi, P.; Oddo, C.M.; Chisci, G.; Frosolini, A.; Gennaro, P.; Abbate, V.; Prattichizzo, D.; Gabriele, G. The Use of Tactile Sensors in Oral and Maxillofacial Surgery: An Overview. Bioengineering 2023, 10, 765. https://doi.org/10.3390/bioengineering10070765
Navalesi P, Oddo CM, Chisci G, Frosolini A, Gennaro P, Abbate V, Prattichizzo D, Gabriele G. The Use of Tactile Sensors in Oral and Maxillofacial Surgery: An Overview. Bioengineering. 2023; 10(7):765. https://doi.org/10.3390/bioengineering10070765
Chicago/Turabian StyleNavalesi, Pietro, Calogero Maria Oddo, Glauco Chisci, Andrea Frosolini, Paolo Gennaro, Vincenzo Abbate, Domenico Prattichizzo, and Guido Gabriele. 2023. "The Use of Tactile Sensors in Oral and Maxillofacial Surgery: An Overview" Bioengineering 10, no. 7: 765. https://doi.org/10.3390/bioengineering10070765