Liquid Metal-Based Electrode Array for Neural Signal Recording
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Electrode Preparation
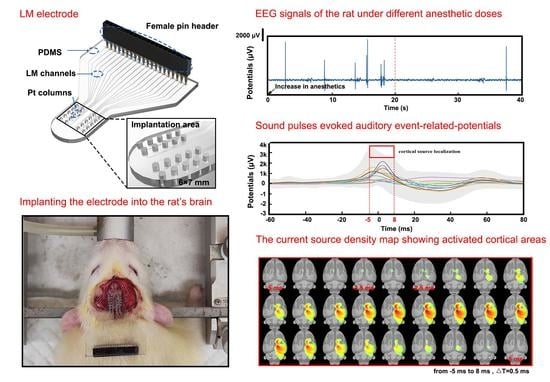
2.2. Design and Fabrication of the LM-Based Electrode
2.3. Characterization of the LM-Based Electrode
2.4. Electrode Implantation Procedure
2.5. EEG Signal Recording and Cortical Source Localization
3. Results and Discussion
3.1. The Flexible Characteristics of the LM-Based Electrode
3.2. The Resistance and Electrochemical Characteristics of the LM-Based Electrode
3.3. Stimulation and Cortical Source Localization after Implantation
4. Conclusions
5. The Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, Y.C.; Lee, H.E.; Shin, A.; Kim, D.G.; Lee, K.J.; Kim, D. Progress in Brain-Compatible Interfaces with Soft Nanomaterials. Adv. Mater. 2020, 32, e1907522. [Google Scholar] [CrossRef]
- Xie, X.; Doloff, J.C.; Yesilyurt, V.; Sadraei, A.; McGarrigle, J.J.; Commis, M.; Veiseh, O.; Farah, S.; Isa, D.; Ghanis, S.; et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2018, 2, 894–906. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef]
- Joo, H.; Lee, Y.; Kim, J.; Yoo, J.; Yoo, S.; Kim, S.; Arya, A.K.; Kim, S.; Choi, S.H.; Lu, N.; et al. Soft implantable drug delivery device integrated wirelessly with wearable devices to treat fatal seizures. Sci. Adv. 2021, 7, eabd4639. [Google Scholar] [CrossRef]
- Hong, G.; Lieber, C.M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019, 20, 330–345. [Google Scholar] [CrossRef]
- Patel, S.R.; Lieber, C.M. Precision electronic medicine in the brain. Nat. Biotechnol. 2019, 37, 1007–1012. [Google Scholar] [CrossRef]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef]
- Jeong, J.; Shin, G.; Park, S.I.; Yu, K.J.; Xu, L.; Rogers, J.A. Soft Materials in Neuroengineering for Hard Problems in Neuroscience. Neuron 2015, 86, 175–186. [Google Scholar] [CrossRef]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Wang, S.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z. Skin-Inspired Electronics: An Emerging Paradigm. Accounts Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Chen, X.; Bao, Z.; Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 2019, 48, 2946–2966. [Google Scholar] [CrossRef]
- Xu, L.; Gutbrod, S.R.; Bonifas, A.P.; Su, Y.; Sulkin, M.S.; Lu, N.; Chung, H.; Jang, K.; Liu, Z.; Ying, M.; et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 2014, 5, 3329. [Google Scholar] [CrossRef]
- Biswas, S.; Sikdar, D.; Das, D.; Mahadevappa, M.; Das, S. PDMS based multielectrode arrays for superior in-vitro retinal stimulation and recording. Biomed. Microdevices 2017, 19, 75. [Google Scholar] [CrossRef]
- Du, M.; Guan, S.; Gao, L.; Lv, S.; Yang, S.; Shi, J.; Wang, J.; Li, H.; Fang, Y. Flexible Micropillar Electrode Arrays for In Vivo Neural Activity Recordings. Small 2019, 15, 1900582. [Google Scholar] [CrossRef]
- Hu, J.; Hossain, R.F.; Navabi, Z.S.; Tillery, A.; Laroque, M.; Donaldson, P.D.; Swisher, S.L.; Kodandaramaiah, S.B. Fully desktop fabricated flexible graphene electrocorticography (ECoG) arrays. J. Neural Eng. 2023, 20, 016019. [Google Scholar] [CrossRef]
- Zhang, S.; Srinivas, M.; Sun, T.; Cheng, M.Y.; Gu, Y.; Lee, C. Development of silicon electrode neural probe and acute study on implantation mechanics. In Proceedings of the 2015 IEEE International Conference on Electron Devices and Solid-State Circuits (EDSSC), Singapore, 1–4 June 2015; pp. 683–686. [Google Scholar]
- Parpura, V. Carbon nanotubes on the brain. Nat. Nanotechnol. 2008, 3, 384–385. [Google Scholar] [CrossRef]
- Wang, K.; Frewin, C.L.; Esrafilzadeh, D.; Yu, C.; Wang, C.; Pancrazio, J.J.; Romero Ortega, M.; Jalili, R.; Wallace, G. High-Performance Graphene-Fiber-Based Neural Recording Microelectrodes. Adv. Mater. 2019, 31, 1805867. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Ayers, H.R.; Majidi, C.; Dickey, M.D. Methods to pattern liquid metals. J. Mater. Chem. C 2015, 3, 3834–3841. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Fu, H.; Lee, J.; Su, J.; Hwang, K.; Rogers, J.A.; Huang, Y. Buckling in serpentine microstructures and applications in elastomer-supported ultra-stretchable electronics with high areal coverage. Soft Matter 2013, 9, 8062. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, L.; Jin, C.; Liu, J. Liquid Metal as Connecting or Functional Recovery Channel for the Transected Sciatic Nerve. arXiv 2014, arXiv:1404.5931. [Google Scholar]
- Liu, F.; Yu, Y.; Yi, L.; Liu, J. Liquid metal as reconnection agent for peripheral nerve injury. Sci. Bull. 2016, 61, 939–947. [Google Scholar] [CrossRef]
- Yi, L.; Liu, J. Liquid metal biomaterials: A newly emerging area to tackle modern biomedical challenges. Int. Mater. Rev. 2017, 62, 415–440. [Google Scholar] [CrossRef]
- Guo, R.; Liu, J. Implantable liquid metal-based flexible neural microelectrode array and its application in recovering animal locomotion functions. J. Micromech. Microeng. 2017, 27, 104002. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, C.; Liu, B.; Jiang, C.; Wang, L.; Zhang, X.; Huang, Q.; Liu, J.; Li, L. Towards an artificial peripheral nerve: Liquid metal-based fluidic cuff electrodes for long-term nerve stimulation and recording. Biosens. Bioelectron. 2022, 216, 114600. [Google Scholar] [CrossRef]
- Dong, R.; Wang, L.; Hang, C.; Chen, Z.; Liu, X.; Zhong, L.; Qi, J.; Huang, Y.; Liu, S.; Wang, L.; et al. Printed Stretchable Liquid Metal Electrode Arrays for In Vivo Neural Recording. Small 2021, 17, 2006612. [Google Scholar] [CrossRef]
- Dong, R.; Liu, X.; Cheng, S.; Tang, L.; Chen, M.; Zhong, L.; Chen, Z.; Liu, S.; Jiang, X. Highly Stretchable Metal–Polymer Conductor Electrode Array for Electrophysiology. Adv. Healthc. Mater. 2021, 10, 2000641. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, R.; Li, L.; Zhang, L.; Liu, B.; Deng, Z.; Wang, L.; Gui, L. A liquid metal based capacitive soft pressure microsensor. Lab Chip 2019, 19, 807–814. [Google Scholar] [CrossRef]
- Ye, Z.; Li, Q.; Zhang, R.; Zhangab, P.; Gui, L. Fabrication of a thin PDMS film with complex liquid metal electrodes embedded and its application as skin sensors. Rsc. Adv. 2022, 12, 8290–8299. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, J.; Liu, M.; Sun, X.; Li, Q.; Cao, L.; Ye, Z.; Gong, J.; He, Z.; Gui, L. Liquid-Metal-Based Stretchable Dual-Parameter Sensor for Simultaneous Detection of Deformation and Temperature. Adv. Mater. Technol. US 2022, 8, 2201264. [Google Scholar] [CrossRef]
- Gramfort, A.; Papadopoulo, T.; Olivi, E.; Clerc, M. OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomed. Eng. Online 2010, 9, 45. [Google Scholar] [CrossRef]
- Kybic, J.; Smutek, D. Computational elastography from standard ultrasound image sequences by global trust region optimization. In International Conference on Information Processing in Medical Imaging; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Litvak, V.; Mattout, J.; Kiebel, S.; Phillips, C.; Henson, R.; Kilner, J.; Barnes, G.; Oostenveld, R.; Daunizeau, J.; Flandin, G. EEG and MEG Data Analysis in SPM8. Comput. Intell. Neurosci. 2011, 2011, 852961. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar]
- Kim, H.; Maleki, T.; Wei, P.; Ziaie, B. A Biaxial Stretchable Interconnect with Liquid-Alloy-Covered Joints on Elastomeric Substrate. J. Microelectromech. Syst. 2009, 18, 138–146. [Google Scholar] [CrossRef]
- Morley, N.B.; Burris, J.; Cadwallader, L.C.; Nornberg, M.D. GaInSn usage in the research laboratory. Rev. Sci. Instrum. 2008, 79, 56107. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, B.; Gao, J.; Lang, Y.; Lv, X.; Deng, Z.; Gui, L.; Liu, J.; Tang, R.; Li, L. Liquid Metal-Based Electrode Array for Neural Signal Recording. Bioengineering 2023, 10, 578. https://doi.org/10.3390/bioengineering10050578
Zhang X, Liu B, Gao J, Lang Y, Lv X, Deng Z, Gui L, Liu J, Tang R, Li L. Liquid Metal-Based Electrode Array for Neural Signal Recording. Bioengineering. 2023; 10(5):578. https://doi.org/10.3390/bioengineering10050578
Chicago/Turabian StyleZhang, Xilong, Bingxin Liu, Jingru Gao, Yiran Lang, Xiaodong Lv, Zhongshan Deng, Lin Gui, Jing Liu, Rongyu Tang, and Lei Li. 2023. "Liquid Metal-Based Electrode Array for Neural Signal Recording" Bioengineering 10, no. 5: 578. https://doi.org/10.3390/bioengineering10050578