3.1. CFD for Shake Flasks
In order to estimate the spatial discretisation error on the one hand and to perform economic CFD simulations on the other hand, a mesh study was carried out. This was quantitatively performed using the grid convergence index (GCI) method, which corresponds to the Richardson extrapolation with a safety factor of
[
74,
75,
76]. This method is considered to be the best practice and is recommended by the OECD [
77]. A detailed explanation of the procedure can be found in [
78,
79,
80]. Five computational meshes with 0.28
to 2.09
were created for the studies, resulting in 3 GCI cases (
Table 2). In each case, the mesh refinement factor
r ranged between
and
[
74]. A safety factor
of
was used for the investigations [
78,
81,
82]. The investigated criterion chosen was the mean Kolmogorov length, which occurs during one complete shaking period.
Table 2 shows the results for the investigations of the baffled shake flask. As can be deduced from the quotients
, the solutions of meshes 3 to 5 are in the asymptotic region of convergence (
represents the formal order of accuracy). Because the relative error
between mesh 4 and 5 was only
but the simulation time increased by
, mesh 4 was used for further investigations. The same investigations were carried out for the shake flask without baffles, again using a mesh with
cells.
The experimental determination of the specific power input in orbitally shaken systems can only be achieved using a very complex setup. However, Büchs et al. [
43] carried out experimental investigations by means of torque measurement and determined an empirical derivation of the specific power input [
43,
83,
84]. The power input for shake flasks, both with and without baffles, is independent of the shaking amplitude as long as they are in phase [
85]. Thus, for the process parameters used here with a working volume of 160
, a shaking rate of 130
, shaking amplitude of 50
and specific power input of
/
(Equation in Büchs et al. [
43]) and
/
(Equation in [
83]) were obtained for the shake flasks without baffles. The shake flask is in phase with a phase number Ph of
and an axial Froude number
of
(Equations (
10) and (
11)) [
85]. The volume of liquid in the shake flask corresponds to
V,
d corresponds to the maximum inner diameter of the shake flask, and
corresponds to the dynamic viscosity of the water phase.
By means of CFD investigations, it was possible to determine the specific power input
via the torque
M acting on the shake flask. Thereby, it is shown that slightly higher values are predicted by Büchs et al. [
83] than by the CFD simulation. A power input of
/
(averaged over a shaking period) is predicted by CFD via the determined torque (Equation (
12)).
The specific power input varies between
/
and
/
over one shaking period. It should be noted, however, that the measured and calculated values of Büchs et al. [
83] scatter significantly (>30%), especially with low specific power inputs. In addition, Büchs et al. [
83] used glass flasks for the investigations, and in the CFD simulations, contact angles were used that correspond to those of polycarbonate (
= 83
,
= 0
to 26
) [
55]. As described in Seidel et al. [
50], the specific power input is dependent on the contact angle and increases with increasing contact angle. For shake flasks with baffles, there is no empirical formula that can be used for validation. However, Peter et al. [
85] described that as long as the shake flasks are in phase, the power input is significantly higher than for shake flasks without baffles under the same process conditions. For shake flasks with baffles, there is also no formula for determining the phase number [
85,
86,
87]. The power input determined by CFD averages
/
over a shaking period and fluctuates between
/
and
/
. Due to the non-rotation symmetrical geometry, the power input fluctuates significantly more over the rotation period than with the shake flask without baffles. This simulation confirmed the statements of Peter et al. [
85] and Li et al. [
88] where the specific power input of shake flasks with baffles is significantly higher than that of shake flasks without baffles. The specific power input can be determined using CFD not only via the torque but also via the energy dissipation rate
(sum of turbulent and viscous energy dissipation rate) [
89]. Because an unstructured mesh was used, where not all mesh cells have the same volume, the local energy dissipation rates
must be multiplied by the corresponding control volume
(and the density of the fluid
) and divided by the total fluid volume
V (Equation (
13)). However, the local energy dissipation rate cannot be directly determined from the simulations carried out as the
k-
-SST turbulence model was used. However, the local energy dissipation rate
corresponds to the product of local turbulent kinetic energy
, local specific dissipation rate
, and model constant
(Equation (
14)).
In this case, a specific power input of only
/
instead of
/
is determined for the shake flask without baffles. This underestimation of the specific power input is typical for the approach that utilises the energy dissipation rate. Multiple authors have shown that the power input determined by this method is up to
lower than that determined via torque [
90,
91,
92,
93]. Tianzhong et al. [
94] described the ratio of specific power input determined via torque to volume-averaged energy dissipation rate (
), which shows a linear dependence that corresponds to
. This ratio practically corresponds to that of these simulations, where the ratio was
for the shake flasks without baffles and
for those with baffles.
Orbitally shaken systems are characterised by their low hydrodynamic heterogeneity
, whereby
is the spacial maximum energy dissipation rate [
40,
95]. Liu et al. [
96] investigated this for both shake flasks with and without baffles using CFD. Thereby, the hydrodynamic heterogeneity for the unbaffled flasks was between
and
and between
and
for the baffled flasks. Peter et al. [
97] experimentally investigated the hydrodynamic heterogeneity in baffled and unbaffled shake flasks by determining the maximum stable droplet diameter, whereby values of up to about 15 were obtained, with the majority of the investigations showing values between 1 and 6. The hydrodynamic heterogeneities determined in this work are consistent with those of Liu et al.’s [
96] work and tend to be minimally higher than the values of Peter et al. [
97]. The local Kolmogorov length
is directly dependent on the local energy dissipation rate (Equation (
15)). In order to calculate a volume-averaged Kolmogorov length
, the sum of the individual local Kolmogorov lengths
multiplied by the control volumes
is formed analogous to Equation (
13) and divided by the liquid volume
V. This volume-averaged Kolmogorov length is
for the shake flasks with baffles and
for the shake flasks without baffles, evaluated over one shaking period. Both values are significantly higher than the determined cell diameter of HEK293 cells (
Section 3.2). These high values in combination with the low hydrodynamic heterogeneity suggest that the cells are not expected to be damaged by the hydrodynamic stress [
38,
98,
99]. The hydrodynamic difference between the two shake flasks investigated can be illustrated by means of a vortex visualisation. For this purpose, the widely used
Q-criterion was used, which corresponds to the second invariant of the velocity gradient tensor (Equation (
16)) [
100,
101,
102]. Positive
Q values correspond to areas where vorticity dominates over viscous stress [
100].
Figure 4 shows the two shake flasks at the same time step with the liquid indicated by shading. A value of 1000
was used as the
Q-criterion. This shows that, compared with the shake flask with baffles, the shake flask without baffles has almost no areas with
. In the case of the shake flask with baffles, it can be seen that vortex regions form around the four baffles. The fluid velocity is at its maximum near the wall on the ridge of the baffles.
3.2. Cultivations in Shake Flasks
The cultivations in the shake flasks lasted for 192 h. Similar to the cultivations in the Minifors 2, peak cell density was reached after a cultivation time of 120 h, with a maximum VCD of
in the baffled shake flasks. The maximum VCD in the unbaffled shake flasks was
. In the shake flasks without baffles, a metabolism shift took place. HEK293 cells metabolise lactate and glucose concomitantly under certain environmental conditions. Martínez-Monge et al. [
103] describe that when lactate and extracellular proteins accumulate, cells start consuming lactate and glucose concomitantly. The cells started to metabolise lactate after a cultivation time of 120
. At this time, the glucose concentration was
/
. The glucose and lactate concentrations at the end of the process were
/
and
/
. In the shake flasks with baffles, the lactate concentration decreased from
/
to
/
between
and
. However, the lactate concentration increased to
/
at the end of the cultivation.
Comparing the maximum VCDs between the two shake flask configurations shows that the VCD
of
for shake flasks with baffles is higher than that of the one for shake flasks without baffles (
). The null hypothesis
of a normal distribution of the VCD
values could not be rejected by the Shapiro–Wilk test for both configurations (significance level
) [
104]. The homoscedasticity tests (Levene and Bartlett) were also unable to reject the
hypothesis of homoscedasticity (
Table 3) [
105,
106]. The Student’s
t-test with the
hypothesis
shows that there is a statistically significant difference between the two configurations in terms of maximum VCD (
,
). To ensure that the statistically significant difference in mean VCD
does not result from an oxygen limitation in the shake flasks without baffles, the theoretical maximum cell density up to an oxygen limitation was determined. For this purpose, the formula for maximum oxygen transfer rate
described by Meier et al. [
107] was used. The measured osmolality of the FreeStyle
TM 293 medium was
. If oxygen transfer rate (OTR) and oxygen uptake rate (OUR) are in equilibrium, the specific oxygen uptake rate
can be used to determine the theoretical maximum VCD (Equations (
17) and (
18)) [
108].
corresponds to the dissolved oxygen concentration at the gas–liquid interphase,
to the dissolved oxygen concentration in the liquid bulk, and
to the biomass concentration.
The maximum specific oxygen uptake rate of
mol
for HEK293 described in the literature was used as the specific oxygen uptake rate [
109,
110]. In order to calculate the theoretical solubility of oxygen, the simplified assumption was made that oxygen was dissolved in water and calculated according to Pappenreiter et al. [
111] and Tromans [
112]. Thus, a volumetric oxygen mass transfer coefficient
value of 16
results in a theoretical oxygen supplementation that is suitable for a cell density of
, which is significantly higher than the cell density reached in all experiments. This calculation is only an approximation, and phenomena such as the biological enhancement factor and other limitations have not been taken into account [
113].
A significant difference is visible in the aggregate size distribution. As can be seen in
Figure 5A, in the cultivations using shake flasks with baffles, an average of
of the viable cells are present as single cells at the time of reaching the maximum VCD. This is significantly more than the
in the cultivations of the shake flasks without baffles. In the literature, aggregate size distributions are often related to the aggregate diameter [
114,
115,
116,
117]. In general, cluster size distributions can be described by a discrete log-normal distribution [
118]. Mendes et al. [
119] describes the cluster size distribution for monolayers for head and neck cancer-5 (HN-5), human epithelioma-2 (HEp-2) and Madin–Darby canine kidney (MDCK) cells using power law distribution. However, this has the disadvantage of the model parameters having to be determined for each distribution.
Figure 5B,C show that the cluster size distribution for the shake flasks both with and without baffles follow a geometric distribution (Equation (
19)). The parameter
p describing the distribution corresponds to the fraction of cells that are not present as aggregates, with
n corresponding to the aggregate size. Thus,
p corresponds to
for the shake flasks without baffles and
for those with baffles. If the maximum likelihood estimation
for the available data is used instead of the fraction of non-aggregated cells,
would be
and
, respectively. The relative differences of the parameter
p are
for shake flasks without baffles and
for shake flasks with baffles.
There are several statistical tests to investigate the goodness-of-fit for geometric distributions [
120,
121,
122]. If the widely used
test is used to determine the goodness-of-fit, (
: cluster size distribution is geometrically distributed), it is shown with
p-value
that the cluster size distribution is statistically significantly different from a geometric distribution with
(
, number of HEK293 cells
,
). The same can be observed with the G test (log-likelihood-ratio) and for the shake flasks with baffles (
,
,
). The detection of a statistically significant difference can be expected with such a large sample number because the statistical power of the test is extremely high [
123,
124]. Due to the high sample size, comparing the cluster size distributions predicted by the geometric distribution with the measured ones shows a statistically significant difference, but this has no practical relevance (
Figure 5B,C).
In addition to the aggregate size distribution, the cell size distribution can also be analysed (
Figure 6). Maschke et al. [
38] describe a normally distributed cell size for CHO XM111-10 with a mean cell diameter of
at the beginning of the exponential growth phase and
at the end. The standard deviation increases from
to
. A normal distribution of cell sizes could also be assumed for the HEK293 cells examined here; however, the quantile–quantile plot showed slightly heavier tails (plots are not shown here). Furthermore, it should be noted that the CedexHiRes analyser only allows for the measurement of cell size distribution in 1
classes. For shake flasks without baffles, the mean cell diameter increases from
(cultivation time
,
,
) to
(
,
,
). For the cultivations in shake flasks with baffles, the mean cell diameter remained constant (
,
,
,
to
,
,
,
). Liu et al. [
21] measured cell diameters of
to
for HEK293 cells with the Cedex AS20 cell counter. Dietmair et al. [
125] measured a mean cell diameter of
for HEK293 cells, and Blumlein et al. [
126] assumed a diameter of 15
. All of the values are within the range of the values measured here.
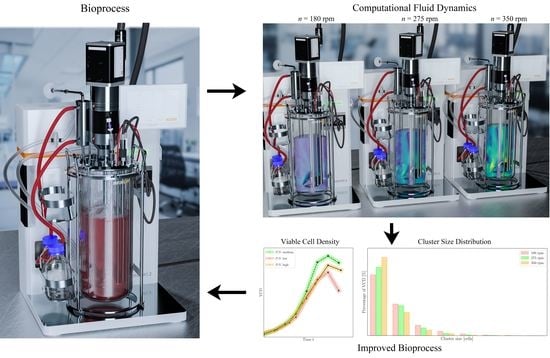
3.3. CFD for Stirred Bioreactor
As for the CFD investigations with the shake flasks, a mesh study was also carried out for the Minifors 2 using the GCI approach. Four meshes and thus two GCI cases were distinguished (
Table 4). Again, care was taken to ensure that
and the volume-averaged Kolmogorov length
were used as the GCI criteria. The results in
Table 4 show that the quotient
is close to one only for the second case, which indicates an asymptotic approximation. Due to the small relative deviation of
, computational mesh 3 with
cells was used for further investigations.
In order to not only quantify the spatial discretisation error but also to validate the model, PIV measurements were performed.
Figure 7A shows the 2D velocity field of the CFD simulation at 180
. By using the 2D velocity field, the simulation becomes comparable to the 2D-2C PIV (
Figure 7B).
Figure 7C shows the velocity profile over the normalised radial distance
(at the level of the red line,
above the bioreactor bottom;
Figure 7A,B). It can be seen that the 2D velocity magnitude between CFD and PIV agrees well. Larger deviations only occur directly at the stirrer. Here, significantly higher velocity magnitudes are predicted by the experiments. However, these high values can be traced back to reflections on the stirrer blade (despite the blades being sprayed black). Another indicator is that these values of 2
/
are higher than the theoretical maximum speed of
/
(stirrer tip speed). A further deviation between PIV and CFD can be observed in the upper area near the wall. The reason for the higher velocity magnitudes in the PIV evaluation is that there is a probe at this point behind the measuring plane, which was also sprayed black, but still resulted in reflections at these two points, which affected the measurement. Despite this deviation, which is largely due to the experiment, the CFD model can be considered validated.
If the validated CFD model is used to determine the specific power input, the characteristic pattern for stirred reactors appears, namely, the specific power input being a power function of the stirrer speed [
42]. For the Minifors 2, only power inputs are published, which were also determined using CFD [
127]. As shown in
Figure 8, the specific power inputs determined here correspond to those from the literature [
127]. The specific power input increases from
/
(
N = 100
,
) to
/
(
N = 500
,
). The modified Reynolds number
is defined according to Equation (
20), with
representing the stirrer diameter.
Maschke and Eibl [
127] describe the specific power input for a working volume of 4
with
, with
corresponding to the tip speed. The calculated Newton number Ne (also known as the power number) decreases from
at 100
(
) to 1.80 at 500
(
). The Ne number is calculated according to Equation (
21). The fact that there is no stagnation of the Ne number as the modified
number increases is consistent with the expected behaviour of flows being in the turbulent transition region [
128]. Zhu et al. [
129] were able to experimentally describe an unaerated Ne number of about
for a system with a three-blade elephant ear impeller in a stirred bioreactor with a
working volume (up-pumping direction). Rotondi et al. [
130] were able to show in the Ambr 250 bioreactor (Sartorius AG, Göttingen, Germany) that, depending on the size and angle of the stirrer blades, the Ne numbers lie between
and
for the elephant ear impeller. The elephant ear impeller examined here lies in the range described by Rotondi et al. [
130].
Figure 8.
Calculated specific power input for the Minifors 2 with a 4
working volume. For comparison, the calculations of Maschke and Eibl [
127] were used, which describe the specific power input as a function of the tip speed
for a working volume of 4
(
,
).
Figure 8.
Calculated specific power input for the Minifors 2 with a 4
working volume. For comparison, the calculations of Maschke and Eibl [
127] were used, which describe the specific power input as a function of the tip speed
for a working volume of 4
(
,
).
In contrast to orbitally shaken bioreactor systems, stirred bioreactors are characterised by their high hydrodynamic heterogeneity. Depending on the stirrer used, this lies between
and 400 [
131,
132,
133,
134]. For the Minifors 2, a hydrodynamic heterogeneity of
was determined. If instead of the energy dissipation rate the dependent Kolmogorov length is represented as a volume-related probability density function, the volume fraction can be determined, which has a cell-critical Kolmogorov length.
Figure 9 shows the normalised volume-related probability density function of the Kolmogorov length as a function of the stirrer speed. The solid red line shows the determined mean HEK293 cell diameter. It can be seen that in the investigated range of 11.4
/
to 1155.3
/
(100
to 500
), the critical eddy size based on the Kolmogorov length scale is larger than the mean cell diameter; thus, no damage should appear. However, the aim of the investigations was to increase the hydrodynamic stress and not to use 13
/
to 60
/
, as is typically the case [
69,
70,
71]. By increasing the hydrodynamic stress and decreasing the Kolmogorov length, there was an attempt to shear cell aggregates and thus minimise the typical aggregate formation of HEK293 cells. By integrating the non-normalised frequency density functions of
Figure 9, it can be seen that in the cultivations with 180
, the largest volume fraction has a Kolmogorov length that is above the mean HEK293 cell diameter. The Kolmogorov lengths are lower than
in only
of the 4
working volume. Only a volume fraction of
( 83
) have a Kolmogorov length lower than the size of a three-cell cluster (
). In the 275
cultivations, a volume fraction of
or 311
has a Kolmogorov length lower than
(in
, the Kolmogorov lengths are lower than
) and a volume fraction of 0.121 or
in the 350
cultivations (in
, the Kolmogorov lengths are lower than
).
3.4. Cultivations in Stirred Bioreactor
The batch experiments in the Minifors 2 bioreactor took between 168
and 196
. The maximum VCD differed depending on the set stirrer speed and specific power input, respectively (
Figure 10). Thus, with a stirrer speed of 180
(
), a VCD of
was achieved. With a stirrer speed of 275
(
= 233
/
), the maximum VCD of
was reached with a speed of 350
(
)
. After about 120
, the cultivations reached their maximum VCD with viabilities above
.
The maximum lactate concentrations were between
/
and
/
for all cultivations and were reached one day before the maximum VCD. The highest lactate concentrations were measured at a speed of 350
. As expected, the lactate concentration decreased again towards the end of the cultivation. Henry et al. [
135] determined a specific lactate production rate in the exponential phase of
for HEK293 cells. The increased lactate production of mammalian cells is often associated with increased hydrodynamic stress by some authors. For example, Sorg et al. [
136] showed that lactate production in Chinese hamster ovary (CHO) cells increased and product titer decreased when hydrodynamic stress was too high. Liu et al. [
137] also showed that the specific lactate production rate in HEK293 cells significantly increased in spinner bioreactors at speeds that were too high. Low shear stress was also shown to stimulate HEK293 productivity [
138]. Zhan et al. [
138] provided an overview of gene regulation under high and low shear stress. Not all authors were able to demonstrate increased lactate production during increased hydrodynamic stress [
139]. However, Godoy-Silva et al. [
139] observed a reduction in cell diameter for CHO cells. In the investigations carried out here, neither a significant change in lactate concentration nor a reduction in cell diameter could be observed (
Appendix A Figure A4). The average cell diameter at the time of maximum VCD was
at 180
,
at 275
and
at 350
. A further investigation, which was not the aim of this research, could be carried out by analysing the cytoskeleton. It is known that the cytoskeleton rearrangement is a response to non-lethal hydrodynamic stress [
140]. For example, actin-binding marker antibodies can be used and studied using immunofluorescence analyses. such investigations have already been carried out for adherent endothelial cells and adherent MDCK cells and could also be carried out for further investigations with the HEK293 suspension cells studied here [
141,
142].
The maximum specific growth rates
were achieved in the cultivation period from
24
to 72
and are comparable for all cultivations. This is
for 180
,
for 275
, and
for 350
. The values also reflect what is documented in the literature, where typical maximum specific growth rates for HEK293 cells range from 0.020
to 0.029
[
23,
24,
25,
26]. In order to follow the aggregate formation and cell morphology online, experiments were carried out using the iLine F probe.
Figure 11 shows exemplary sections from the online recording of the holographic images. The optical height
h of the 3D image was reconstructed using OsOne software through a Fourier transformation [
67]. At timepoints
and
, two extreme forms of clusters with three cells are marked, which were also used for the assessment of the Kolmogorov length distribution in
Section 3.3. Such linear and spherical cell clusters are also described in the literature [
143].
Figure 12A shows the temporal development of the VCD and aggregation during the cultivation in Minifors 2 at a stirrer speed of 180
. Both the daily offline measured values and those of the online iLineF system are presented. It becomes evident that the values measured offline correspond well with the values measured online over the entire cultivation period. Altenburg et al. [
144] showed similar accuracies between the VCD determination using iLineF and offline determination in cultivations with insect cells. The cell aggregation rate correlates with the VCD. Both values increase until the time of
and then decrease again until the end of the cultivation. The increase in aggregation with increasing cell density is also described in the literature [
145].
Figure 12B shows the lactate and glucose concentrations measured offline for the same cultivation.
It can be seen that the cell diameter does not change significantly with different specific power inputs, but the aggregation range is strongly dependent on the specific power input.
Figure 13 shows the cluster size distribution similar to the experiments with the shake flasks. In
Figure 13A, the difference in aggregation at the time of the maximum VCD becomes clearly visible. At a stirrer speed of 350
, only
of the viable cells are present as aggregates, whereas it is already at
for a stirrer speed of 275
and
at 180
. The cluster size distribution also precisely follows a geometric distribution for the cultivations in the stirred bioreactor, as was already the case for the cultivations with the shake flasks. Here, too, the proportion of non-aggregated cells is suitable as a parameter
p of the geometric distribution. As shown in
Figure 13B to
Figure 13D, the parameter
p determined in this way at 350
deviates by only
from the maximum likelihood estimated parameter
(
at 275
and
at 180
).
When the proportion of non-aggregated cells is expressed as a function of the volume-averaged Kolmogorov length scale, a linear relationship can be observed irrespective of the cultivation system and the type of mechanical power input (
Figure 14). This insight now allows for the use of the mean Kolmogorov length scale determined by CFD to predict the aggregate size distribution at the time of maximum VCD. The linear relationship described in
Figure 14 can thus be substituted in Equation (
19), which reflects the direct relationship between the aggregate size distribution and mean Kolmogorov length scale (Equation (
22)).