Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix
Abstract
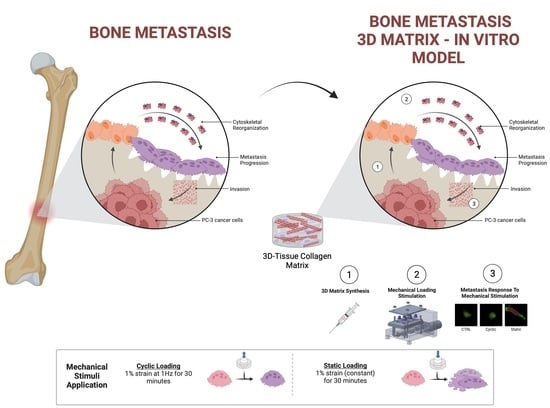
:1. Introduction
2. Materials and Methods
2.1. Preparation of Prostate Cancer Cell-Embedded 3D Collagen Tissue Matrix
2.2. Mechanical Stimuli Application System to 3D PC-3-Collagen Tissue Matrix: The EQUicycler
2.3. Cytoskeletal Organization of PC-3 Cells in Response to Mechanical Stimuli within the 3D Tissue Matrix
2.4. Changes in Cell Elongation Index of PC-3 Cells in Response to Mechanical Stimuli within the 3D Tissue Matrix
2.5. Proliferation of PC-3 Cells in Response to Mechanical Stimuli within the 3D Tissue Matrix
2.6. Changes in Invasion Capacity of PC-3 Cells in Response to Mechanical Stimuli within the 3D Tissue Matrix
2.7. Changes in Gene Expression of PC-3 Cells in Response to Mechanical Stimuli within the 3D Tissue Matrix
2.8. Statistical Methods
3. Results
3.1. Mechanical Stimuli within the 3D Tissue Matrix Mediate Cytoskeletal Reorganization of PC-3 Cells
3.2. Mechanical Stimuli Mediate PC-3 Cell Morphology within the 3D Tissue Matrix
3.3. Altered Actin Dynamics in PC-3 Cells within the 3D Tissue Matrix Are Dependent on the Type of Mechanical Stimuli
3.4. Proliferation of PC-3 Cells in Response to Mechanical Stimuli
3.5. The Changes in Expression of Metastasis-Associated Genes in Response to Mechanical Stimuli
3.6. Mechanical Stimuli Mediate Invasion Capacity of PC-3 Cells to the Adjacent Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Li, X.; Du, Y.; Li, X. Decoding the past and future of breast cancer bone metastasis: A bibliometric analysis from 2003 to 2022. Asian J. Surg. 2023, ahead of print. [Google Scholar] [CrossRef]
- Thilakan, A.T.; Nandakumar, N.; Balakrishnan, A.R.; Pooleri, G.K.; Nair, S.V.; Sathy, B.N. Development and characterisation of suitably bioengineered microfibrillar matrix-based 3D prostate cancer model forin vitrodrug testing. Biomed. Mater. 2023, 18, 065016. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; King, H.; Shah, N.; Wang, K.; Rodriguez-Teja, M.; Gronau, J.H.; Waxman, J.; Sturge, J. Tumor-associated Endo180 requires stromal-derived LOX to promote metastatic prostate cancer cell migration on human ECM surfaces. Clin. Exp. Metastasis 2016, 33, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.; Latif, A.; Williams, K.J.; Mancuso, E.; Tirella, A. Invasion and Secondary Site Colonization as a Function of In Vitro Primary Tumor Matrix Stiffness: Breast to Bone Metastasis. Adv. Healthc. Mater. 2023, 12, e2201898. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.A.; Cooper, C.R.; Sikes, R.A. Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod. Biol. Endocrinol. 2004, 2, 2. [Google Scholar] [CrossRef]
- Windus, L.C.E.; Matigian, N.; Avery, V.M. Induction of Reactive Bone Stromal Fibroblasts in 3D Models of Prostate Cancer Bone Metastases. Biology 2023, 12, 861. [Google Scholar] [CrossRef]
- Yao, D.; Qiao, F.; Song, C.; Lv, Y. Matrix stiffness regulates bone repair by modulating 12-lipoxygenase-mediated early inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112359. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Rowley, D.R. Reactive stroma in prostate cancer progression. J. Urol. 2001, 166, 2472–2483. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Smith, M.J.; Smith, V.C.; Dang, T.D.; Rowley, D.R. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 2002, 8, 2912–2923. [Google Scholar]
- Elsaadany, M.; Harris, M.; Yildirim-Ayan, E. Design and Validation of Equiaxial Mechanical Strain Platform, EQUicycler, for 3D Tissue Engineered Constructs. Biomed. Res. Int. 2017, 2017, 3609703. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S. Cancer treatment and survivorship statistics, 2012. CA A Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Zhu, H.; Lin, Q.; Yu, Z. Risk Evaluation of Bone Metastases and a Simple Tool for Detecting Bone Metastases in Prostate Cancer: A Population-Based Study. Comput. Math. Methods Med. 2023, 2023, 9161763. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Z.; Makarov, D.; Li, X. Current treatments and novel therapeutic targets for castration resistant prostate cancer with bone metastasis. Am. J. Clin. Exp. Urol. 2013, 1, 30–38. [Google Scholar] [PubMed]
- Nørgaard, M.; Jensen, A.Ø.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sørensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.L.; Davy, D.T.; Keaveny, T.M. Orthopaedic Biomechanics; Pearson/Prentice Hall: Hoboken, NJ, USA, 2006. [Google Scholar]
- Tzaphlidou, M. Bone architecture: Collagen structure and calcium/phosphorus maps. J. Biol. Phys. 2008, 34, 39–49. [Google Scholar] [CrossRef]
- Sieh, S.; Lubik, A.A.; Clements, J.A.; Nelson, C.C.; Hutmacher, D.W. Interactions between human osteoblasts and prostate cancer cells in a novel 3D in vitro model. Organogenesis 2010, 6, 181–188. [Google Scholar] [CrossRef]
- Mognetti, B.; La Montagna, G.; Perrelli, M.G.; Pagliaro, P.; Penna, C. Bone marrow mesenchymal stem cells increase motility of prostate cancer cells via production of stromal cell-derived factor-1α. J. Cell. Mol. Med. 2013, 17, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhau, H.E.; Odero-Marah, V.; Lue, H.W.; Nomura, T.; Wang, R.X.; Chu, G.; Liu, Z.R.; Zhou, B.P.; Huang, W.C.; Chung, L.W.K. Epithelial to mesenchymal transition (EMT) in human prostate cancer: Lessons learned from ARCaP model. Clin. Exp. Metastasis 2008, 25, 601–610. [Google Scholar] [CrossRef]
- Freeman, M.R.; Li, Q.L.; Chung, L.W.K. Can Stroma Reaction Predict Cancer Lethality? Clin. Cancer Res. 2013, 19, 4905–4907. [Google Scholar] [CrossRef]
- Josson, S.; Matsuoka, Y.; Chung, L.W.K.; Zhau, H.Y.E.; Wang, R.X. Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin. Cell Dev. Biol. 2010, 21, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.; Lynch, C.C.; Fleming, J.; Hamming, D.; Martin, M.D.; Schwartz, H.S.; Matrisian, L.M.; Holt, G.E. The application of a murine bone bioreactor as a model of tumor: Bone interaction. Clin. Exp. Metastasis 2006, 23, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, K.; Peng, Y.; Wu, W.; Chen, F.; Shao, Z.; Zhang, Z. Animal models of cancer metastasis to the bone. Front Oncol. 2023, 13, 1165380. [Google Scholar] [CrossRef]
- Reichert, J.C.; Quent, V.M.C.; Burke, L.J.; Stansfield, S.H.; Clements, J.A.; Hutmacher, D.W. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials 2010, 31, 7928–7936. [Google Scholar] [CrossRef]
- Elsaadany, M.; Winters, K.; Adams, S.; Stasuk, A.; Ayan, H.; Yildirim-Ayan, E. Equiaxial Strain Modulates Adipose-derived Stem Cell Differentiation within 3D Biphasic Scaffolds towards Annulus Fibrosus. Sci. Rep. 2017, 7, 12868. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Stasuk, A.; Elsaadany, M.; Yildirim-Ayan, E. Effect of Uniaxial Tensile Cyclic Loading Regimes on Matrix Organization and Tenogenic Differentiation of Adipose-Derived Stem Cells Encapsulated within 3D Collagen Scaffolds. Stem Cells Int. 2017, 2017, 6072406. [Google Scholar] [CrossRef] [PubMed]
- Thibaudeau, L.; Taubenberger, A.; Reichert, V.; Hutmacher, D. Studying the drug-responsiveness of breast cancer cells cultured within human osteoblast-derived matrices. J. Tissue Eng. Regen. Med. 2012, 6, 354. [Google Scholar]
- Pathi, S.P.; Kowalczewski, C.; Tadipatri, R.; Fischbach, C. A Novel 3-D Mineralized Tumor Model to Study Breast Cancer Bone Metastasis. PLoS ONE 2010, 5, e8849. [Google Scholar] [CrossRef]
- Wyckoff, J.B.; Jones, J.G.; Condeelis, J.S.; Segall, J.E. A critical step in metastasis: In vivo analysis of intravasation at the primary tumor. Cancer Res. 2000, 60, 2504–2511. [Google Scholar]
- Pathi, S.P.; Lin, D.D.W.; Dorvee, J.R.; Estroff, L.A.; Fischbach, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 2011, 32, 5112–5122. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Wuescher, L.M.; Worth, R.; Yildirim-Ayan, E. Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization. Ann. Biomed. Eng. 2019, 47, 2213–2231. [Google Scholar] [CrossRef]
- Dozmorov, M.G.; Hurst, R.E.; Culkin, D.J.; Kropp, B.P.; Frank, M.B.; Osban, J.; Penning, T.M.; Lin, H.K. Unique patterns of molecular profiling between human prostate cancer LNCaP and PC-3 cells. Prostate 2009, 69, 1077–1090. [Google Scholar] [CrossRef]
- Cooper, C.R.; Chay, C.H.; Gendernalik, J.D.; Lee, H.L.; Bhatia, J.; Taichman, R.S.; McCauley, L.K.; Keller, E.T.; Pienta, K.J. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 2003, 97 (Suppl. S3), 739–747. [Google Scholar] [CrossRef]
- Jacho, D.; Rabino, A.; Garcia-Mata, R.; Yildirim-Ayan, E. Mechanoresponsive regulation of fibroblast-to-myofibroblast transition in three-dimensional tissue analogues: Mechanical strain amplitude dependency of fibrosis. Sci. Rep. 2022, 12, 16832. [Google Scholar] [CrossRef] [PubMed]
- Trumbull, A.; Subramanian, G.; Yildirim-Ayan, E. Mechanoresponsive musculoskeletal tissue differentiation of adipose-derived stem cells. Biomed. Eng. Online 2016, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.M. Compressive Stress Enhances Coordinated Migration of Mammary Carcinoma Cells; Massachusetts Institute of Technology: Cambridge, MA, USA, 2010. [Google Scholar]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Turner, C.H. Effects of loading frequency on mechanically induced bone formation. J. Bone Miner. Res. 2001, 16, 918–924, Erratum in J. Bone Miner. Res. 2002, 17, 182. [Google Scholar] [CrossRef]
- Takano, Y.; Turner, C.H.; Burr, D.B. Elastic anisotropy of osteonal bone is dependent on the mechanical strain distribution. J. Bone Miner. Res. 1996, 11, M347. [Google Scholar]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the Functional-Response of Bone to Mechanical Strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef]
- Turner, C.H. Is Yield Strain in Cancellous Bone Isotropic? J. Biomech. 1995, 28, 763. [Google Scholar] [CrossRef] [PubMed]
- Shieh, A.C. Biomechanical Forces Shape the Tumor Microenvironment. Ann. Biomed. Eng. 2011, 39, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; He, W.; Lin, N.; Wang, X.; Fan, Q.X. N-cadherin knock-down decreases invasiveness of esophageal squamous cell carcinoma in vitro. World J. Gastroenterol. 2009, 15, 697–704. [Google Scholar] [CrossRef]
- Jaggi, M.; Nazemi, T.; Abrahams, N.A.; Baker, J.J.; Galich, A.; Smith, L.M.; Balaji, K.C. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate 2006, 66, 193–199. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Yamada, S. N-Cadherin Dependent Collective Cell Invasion of Prostate Cancer Cells Is Regulated by the N-Terminus of alpha-Catenin. PLoS ONE 2013, 8, e55069. [Google Scholar] [CrossRef]
- Tomita, K.; van Bokhoven, A.; van Leenders, G.J.L.H.; Ruijter, E.T.G.; Jansen, C.F.J.; Bussemakers, M.J.G.; Schalken, J.A. Cadherin switching in human prostate cancer progression. Cancer Res. 2000, 60, 3650–3654. [Google Scholar] [PubMed]
- Rao, J.Y.; Li, N. Microfilament actin remodeling as a potential target for cancer drug development. Curr. Cancer Drug Targets 2004, 4, 345–354. [Google Scholar] [CrossRef]
- Mogilner, A.; Oster, G. Cell motility driven by actin polymerization. Biophys. J. 1996, 71, 3030–3045. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Yamazaki, D.; Kurisu, S.; Takenawa, T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005, 96, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Demou, Z.N. Gene expression profiles in 3D tumor analogs indicate compressive strain differentially enhances metastatic potential. Ann. Biomed. Eng. 2010, 38, 3509–3520. [Google Scholar] [CrossRef] [PubMed]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Androulidaki, A.; Dermitzaki, E.; Venihaki, M.; Karagianni, E.; Rassouli, O.; Andreakou, E.; Stournaras, C.; Margioris, A.N.; Tsatsanis, C. Corticotropin Releasing Factor promotes breast cancer cell motility and invasiveness. Mol. Cancer 2009, 8, 30. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, S.; Guise, T.A.; Chirgwin, J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell Endocrinol. 2009, 310, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hao, J.; Mao, Y.; Jin, Z.Q.; Cao, R.; Zhu, C.H.; Liu, X.H.; Liu, C.; Ding, X.L.; Wang, X.D.; et al. bFGF Promotes Migration and Induces Cancer-Associated Fibroblast Differentiation of Mouse Bone Mesenchymal Stem Cells to Promote Tumor Growth. Stem Cells Dev. 2016, 25, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef]
- Edmonds, B.T.; Wyckoff, J.; Yeung, Y.-G.; Wang, Y.; Stanley, E.R.; Jones, J.; Segall, J.; Condeelis, J. Elongation factor-1 alpha is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma. J. Cell Sci. 1996, 109, 2705–2714. [Google Scholar] [CrossRef]
- Owen, L.M.; Adhikari, A.S.; Patel, M.; Grimmer, P.; Leijnse, N.; Kim, M.C.; Notbohm, J.; Franck, C.; Dunn, A.R. A cytoskeletal clutch mediates cellular force transmission in a soft, three-dimensional extracellular matrix. Mol. Biol. Cell 2017, 28, 1959–1974. [Google Scholar] [CrossRef]
- Gayer, C.P.; Basson, M.D. The effects of mechanical forces on intestinal physiology and pathology. Cell. Signal. 2009, 21, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Pokorna, E.; Jordan, P.W.; Oneill, C.H.; Zicha, D.; Gilbert, C.S.; Vesely, P. Actin Cytoskeleton and Motility in Rat Sarcoma Cell-Populations with Different Metastatic Potential. Cell Motil. Cytoskelet. 1994, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditto, M.; Jacho, D.; Eisenmann, K.M.; Yildirim-Ayan, E. Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix. Bioengineering 2023, 10, 1271. https://doi.org/10.3390/bioengineering10111271
Ditto M, Jacho D, Eisenmann KM, Yildirim-Ayan E. Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix. Bioengineering. 2023; 10(11):1271. https://doi.org/10.3390/bioengineering10111271
Chicago/Turabian StyleDitto, Maggie, Diego Jacho, Kathryn M. Eisenmann, and Eda Yildirim-Ayan. 2023. "Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix" Bioengineering 10, no. 11: 1271. https://doi.org/10.3390/bioengineering10111271