Machine Learning-Based Segmentation of the Thoracic Aorta with Congenital Valve Disease Using MRI
Abstract
:1. Introduction
2. Method
2.1. MRI Acquisition
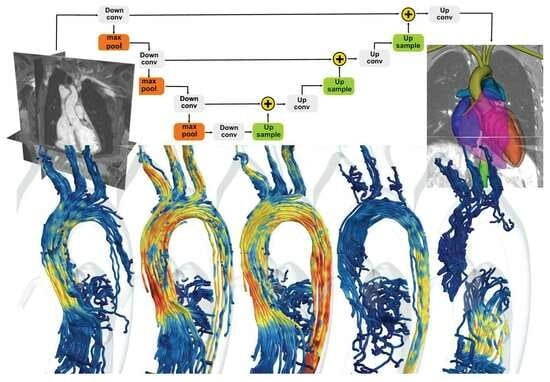
2.2. ML-Based Segmentation
3. Result
3.1. Segmentation Evaluation
3.2. Segmentation Runtime
3.3. Flow Rate, Pulse Wave Velocity, and Arterial Distensibility
3.4. Vortical Structures
3.5. Wall Shear Stress
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tretter, J.T.; Spicer, D.E.; Mori, S.; Chikkabyrappa, S.; Redington, A.N.; Anderson, R.H. The significance of the interleaflet triangles in determining the morphology of congenitally abnormal aortic valves: Implications for noninvasive imaging and surgical management. J. Am. Soc. Echocardiogr. 2016, 29, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Sundström, E.; Jonnagiri, R.; Gutmark-Little, I.; Gutmark, E.; Critser, P.; Taylor, M.; Tretter, J. Effects of Normal Variation in the Rotational Position of the Aortic Root on Hemodynamics and Tissue Biomechanics of the Thoracic Aorta. Cardiovasc. Eng. Technol. 2020, 11, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sundström, E.; Jonnagiri, R.; Gutmark-Little, I.; Gutmark, E.; Critser, P.; Taylor, M.; Tretter, J. Hemodynamics and tissue biomechanics of the thoracic aorta with a trileaflet aortic valve at different phases of valve opening. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3345. [Google Scholar] [CrossRef] [PubMed]
- Jonnagiri, R.; Sundström, E.; Gutmark, E.; Anderson, S.; Pednekar, A.; Taylor, M.; Tretter, J.; Gutmark-Little, I. Influence of aortic valve morphology on vortical structures and wall shear stress. Med. Biol. Eng. Comput. 2023, 61, 1489–1506. [Google Scholar] [CrossRef]
- Sundström, E.; Tretter, J.T. Impact of Variation in Interleaflet Triangle Height between Fused Leaflets in the Functionally Bicuspid Aortic Valve on Hemodynamics and Tissue Biomechanics. J. Eng. Sci. Med. Diagn. Ther. 2022, 5, 031102. [Google Scholar] [CrossRef]
- Guala, A.; Dux-Santoy, L.; Teixido-Tura, G.; Ruiz-Muñoz, A.; Galian-Gay, L.; Servato, M.L.; Valente, F.; Gutiérrez, L.; González-Alujas, T.; Johnson, K.M.; et al. Wall shear stress predicts aortic dilation in patients with bicuspid aortic valve. Cardiovasc. Imaging 2022, 15, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bech-Hanssen, O.; Svensson, F.; Polte, C.L.; Johnsson, Å.A.; Gao, S.A.; Lagerstrand, K.M. Characterization of complex flow patterns in the ascending aorta in patients with aortic regurgitation using conventional phase-contrast velocity MRI. Int. J. Cardiovasc. Imaging 2018, 34, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Meierhofer, C.; Schneider, E.P.; Lyko, C.; Hutter, A.; Martinoff, S.; Markl, M.; Hager, A.; Hess, J.; Stern, H.; Fratz, S. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: A prospective study. Eur. Heart J.-Imaging 2013, 14, 797–804. [Google Scholar] [CrossRef]
- Saikrishnan, N.; Mirabella, L.; Yoganathan, A.P. Bicuspid aortic valves are associated with increased wall and turbulence shear stress levels compared to trileaflet aortic valves. Biomech. Model. Mechanobiol. 2015, 14, 577–588. [Google Scholar] [CrossRef]
- Dux-Santoy, L.; Guala, A.; Sotelo, J.; Uribe, S.; Teixidó-Turà, G.; Ruiz-Muñoz, A.; Hurtado, D.E.; Valente, F.; Galian-Gay, L.; Gutiérrez, L.; et al. Low and oscillatory wall shear stress is not related to aortic dilation in patients with bicuspid aortic valve: A time-resolved 3-dimensional phase-contrast magnetic resonance imaging study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e10–e20. [Google Scholar] [CrossRef]
- Mahadevia, R.; Barker, A.J.; Schnell, S.; Entezari, P.; Kansal, P.; Fedak, P.W.; Malaisrie, S.C.; McCarthy, P.; Collins, J.; Carr, J.; et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014, 129, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, L.; Barker, A.J.; Saikrishnan, N.; Coco, E.R.; Mangiameli, D.J.; Markl, M.; Yoganathan, A.P. MRI-based protocol to characterize the relationship between bicuspid aortic valve morphology and hemodynamics. Ann. Biomed. Eng. 2015, 43, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.J.; Markl, M.; Bürk, J.; Lorenz, R.; Bock, J.; Bauer, S.; Schulz-Menger, J.; von Knobelsdorff-Brenkenhoff, F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ. Cardiovasc. Imaging 2012, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sekine, T.; Ando, T.; Ishii, Y.; Kumita, S. Utility of 4D flow MRI in thoracic aortic diseases: A Literature Review of Clinical Applications and Current Evidence. Magn. Reson. Med. Sci. 2022, 21, 327–339. [Google Scholar] [CrossRef]
- Adriaans, B.P.; Wildberger, J.E.; Westenberg, J.J.; Lamb, H.J.; Schalla, S. Predictive imaging for thoracic aortic dissection and rupture: Moving beyond diameters. Eur. Radiol. 2019, 29, 6396–6404. [Google Scholar] [CrossRef]
- Hope, T.A.; Markl, M.; Wigström, L.; Alley, M.T.; Miller, D.C.; Herfkens, R.J. Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 26, 1471–1479. [Google Scholar] [CrossRef]
- Wawak, M.; Tekieli, Ł.; Badacz, R.; Pieniążek, P.; Maciejewski, D.; Trystuła, M.; Przewłocki, T.; Kabłak-Ziembicka, A. Clinical Characteristics and Outcomes of Aortic Arch Emergencies: Takayasu Disease, Fibromuscular Dysplasia, and Aortic Arch Pathologies: A Retrospective Study and Review of the Literature. Biomedicines 2023, 11, 2207. [Google Scholar]
- Wasserthal, J.; Meyer, M.; Breit, H.C.; Cyriac, J.; Yang, S.; Segeroth, M. TotalSegmentator: Robust segmentation of 104 anatomical structures in CT images. arXiv 2022, arXiv:2208.05868. [Google Scholar] [CrossRef]
- Berhane, H.; Scott, M.; Elbaz, M.; Jarvis, K.; McCarthy, P.; Carr, J.; Malaisrie, C.; Avery, R.; Barker, A.J.; Robinson, J.D.; et al. Fully automated 3D aortic segmentation of 4D flow MRI for hemodynamic analysis using deep learning. Magn. Reson. Med. 2020, 84, 2204–2218. [Google Scholar] [CrossRef]
- Artzner, C.; Bongers, M.N.; Kärgel, R.; Faby, S.; Hefferman, G.; Herrmann, J.; Nopper, S.L.; Perl, R.M.; Walter, S.S. Assessing the Accuracy of an Artificial Intelligence-Based Segmentation Algorithm for the Thoracic Aorta in Computed Tomography Applications. Diagnostics 2022, 12, 1790. [Google Scholar] [CrossRef]
- Fujiwara, T.; Berhane, H.; Scott, M.B.; Englund, E.K.; Schäfer, M.; Fonseca, B.; Berthusen, A.; Robinson, J.D.; Rigsby, C.K.; Browne, L.P.; et al. Segmentation of the Aorta and Pulmonary Arteries Based on 4D Flow MRI in the Pediatric Setting Using Fully Automated Multi-Site, Multi-Vendor, and Multi-Label Dense U-Net. J. Magn. Reson. Imaging 2022, 55, 1666–1680. [Google Scholar] [CrossRef]
- Sievers, H.H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Georgiou, T.; Lew, M.S. A review of semantic segmentation using deep neural networks. Int. J. Multimed. Inf. Retr. 2018, 7, 87–93. [Google Scholar] [CrossRef]
- Weinland, D.; Ronfard, R.; Boyer, E. A survey of vision-based methods for action representation, segmentation and recognition. Comput. Vis. Image Underst. 2011, 115, 224–241. [Google Scholar] [CrossRef]
- Papandreou, G.; Chen, L.C.; Murphy, K.P.; Yuille, A.L. Weakly-and semi-supervised learning of a deep convolutional network for semantic image segmentation. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015; pp. 1742–1750. [Google Scholar] [CrossRef]
- Caesar, H.; Uijlings, J.; Ferrari, V. Region-based semantic segmentation with end-to-end training. In Proceedings of the Computer Vision—ECCV 2016: 14th European Conference, Amsterdam, The Netherlands, 11–14 October 2016; pp. 381–397. [Google Scholar]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Bearman, A.; Russakovsky, O.; Ferrari, V.; Fei-Fei, L. What’s the point: Semantic segmentation with point supervision. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 11–14 October 2016; pp. 549–565. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Bernard, O.; Lalande, A.; Zotti, C.; Cervenansky, F.; Yang, X.; Heng, P.A.; Cetin, I.; Lekadir, K.; Camara, O.; Ballester, M.A.G.; et al. Deep learning techniques for automatic MRI cardiac multi-structures segmentation and diagnosis: Is the problem solved? IEEE Trans. Med. Imaging 2018, 37, 2514–2525. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S.; Bai, N.; Han, K.; Liu, Q.; Yao, S.; Tang, H.; Zhang, C.; Lu, Z.; Huang, Q.; et al. A deep learning-based auto-segmentation system for organs-at-risk on whole-body computed tomography images for radiation therapy. Radiother. Oncol. 2021, 160, 175–184. [Google Scholar] [CrossRef]
- Sundar, L.K.S.; Yu, J.; Muzik, O.; Kulterer, O.C.; Fueger, B.; Kifjak, D.; Nakuz, T.; Shin, H.M.; Sima, A.K.; Kitzmantl, D.; et al. Fully automated, semantic segmentation of whole-body 18F-FDG PET/CT images based on data-centric artificial intelligence. J. Nucl. Med. 2022, 63, 1941–1948. [Google Scholar] [CrossRef]
- Trägårdh, E.; Borrelli, P.; Kaboteh, R.; Gillberg, T.; Ulén, J.; Enqvist, O.; Edenbrandt, L. RECOMIA—A cloud-based platform for artificial intelligence research in nuclear medicine and radiology. EJNMMI Phys. 2020, 7, 51. [Google Scholar] [CrossRef]
- Evangelista, A.; Gallego, P.; Calvo-Iglesias, F.; Bermejo, J.; Robledo-Carmona, J.; Sánchez, V.; Saura, D.; Arnold, R.; Carro, A.; Maldonado, G.; et al. Anatomical and clinical predictors of valve dysfunction and aortic dilation in bicuspid aortic valve disease. Heart 2018, 104, 566–573. [Google Scholar] [CrossRef]
- Sundström, E.; Tretter, T.J. Impact of Variation in Commissural Angle between Fused Leaflets in the Functionally Bicuspid Aortic Valve on Hemodynamics and Tissue Biomechanics. Bioengineering, 2023; submitted (unpublished). [Google Scholar]
- Sundström, E.; Michael, J.; Najm, H.K.; Tretter, J.T. Blood Speckle Imaging: An Emerging Method for Perioperative Evaluation of Subaortic and Aortic Valvar Repair. Bioengineering 2023, 10, 1183. [Google Scholar] [CrossRef]
- Schäfers, H.J. The 10 commandments for aortic valve repair. Innovations 2019, 14, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, E.; Antiga, L.; Puppini, G.; Quarteroni, A.; Luciani, G.B.; Vergara, C. Helical flows and asymmetry of blood jet in dilated ascending aorta with normally functioning bicuspid valve. Biomech. Model. Mechanobiol. 2013, 12, 801–813. [Google Scholar] [CrossRef]
- Bissell, M.M.; Hess, A.T.; Biasiolli, L.; Glaze, S.J.; Loudon, M.; Pitcher, A.; Davis, A.; Prendergast, B.; Markl, M.; Barker, A.J.; et al. Aortic dilation in bicuspid aortic valve disease: Flow pattern is a major contributor and differs with valve fusion type. Circ. Cardiovasc. Imaging 2013, 6, 499–507. [Google Scholar] [CrossRef]
- Laudato, M.; Mosca, R.; Mihaescu, M. Buckling critical pressures in collapsible tubes relevant for biomedical flows. Sci. Rep. 2023, 13, 9298. [Google Scholar] [CrossRef]
- Sundström, E.; Oren, L.; Farbos de Luzan, C.; Gutmark, E.; Khosla, S. Fluid-Structure Interaction Analysis of Aerodynamic and Elasticity Forces During Vocal Fold Vibration. J. Voice 2022. [Google Scholar] [CrossRef] [PubMed]
- Sundström, E.; Oren, L. Change in aeroacoustic sound mechanism during sibilant sound with different velopharyngeal opening sizes. Med. Biol. Eng. Comput. 2021, 59, 937–945. [Google Scholar] [CrossRef]
- Sundström, E.; Boyce, S.; Oren, L. Effects of velopharyngeal openings on flow characteristics of nasal emission. Biomech. Model. Mechanobiol. 2020, 19, 1447–1459. [Google Scholar] [CrossRef]
- Sundström, E.; Oren, L. Sound production mechanisms of audible nasal emission during the sibilant/s/. J. Acoust. Soc. Am. 2019, 146, 4199–4210. [Google Scholar] [CrossRef]








| Parameter | Aorta (cm2) | Left Ventricle (cm2) |
|---|---|---|
| TAV1 | 1.4 (2.0) [0.82] | 17.4 (20.9) [0.91] |
| TAV2 | 1.3 (1.9) [0.81] | 11.1 (14.2) [0.88] |
| TAV3 | 0.9 (2.1) [0.60] | 18.4 (22.5) [0.90] |
| and of Dice score | [0.72 ± 0.12] | [0.86 ± 0.06] |
| BAV1 | 2.9 (2.2) [0.86] | 24.1 (27.1) [0.94] |
| BAV2 | 2.2 (3.5) [0.75] | 20.8 (29.3) [0.83] |
| BAV3 | 2.7 (3.7) [0.84] | 14.3 (17.8) [0.89] |
| and of Dice score | [0.82 ± 0.06] | [0.89 ± 0.06] |
| Case | Size (Voxels) (mm) | Runtime | RAM | GPU Mem |
|---|---|---|---|---|
| TAV | (320 × 320 × 100) (0.9 × 0.9 × 2.4 mm) | 1 min 48 s | 5.1 GB | 3.0 GB |
| BAV | (400 × 400 × 100) (0.8 × 0.8 × 2.8 mm) | 2 min 2 s | 5.4 GB | 3.2 GB |
| Parameter | TAV | BAV |
|---|---|---|
| Heart rate (bpm) | 70 | 47 |
| Net volume (mL) | 67 | 108 |
| Ascending flow (L/min) | 4.7 | 5.1 |
| Regurgitant fraction (%) | 0.3 | 1.5 |
| Descending flow (L/min) | 3.4 | 3.2 |
| Aortic length (mm) | 112 | 156 |
| PWV time to foot (m/s) | 3.9 | 3.8 |
| Distensibility (1/mmHg) | ||
| 0.3 | 0.27 | |
| (mmHg) | 36 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundström, E.; Laudato, M. Machine Learning-Based Segmentation of the Thoracic Aorta with Congenital Valve Disease Using MRI. Bioengineering 2023, 10, 1216. https://doi.org/10.3390/bioengineering10101216
Sundström E, Laudato M. Machine Learning-Based Segmentation of the Thoracic Aorta with Congenital Valve Disease Using MRI. Bioengineering. 2023; 10(10):1216. https://doi.org/10.3390/bioengineering10101216
Chicago/Turabian StyleSundström, Elias, and Marco Laudato. 2023. "Machine Learning-Based Segmentation of the Thoracic Aorta with Congenital Valve Disease Using MRI" Bioengineering 10, no. 10: 1216. https://doi.org/10.3390/bioengineering10101216