Subcritical Water Pretreatment for the Efficient Valorization of Sorghum Distillery Residue for the Biorefinery Platform
Abstract
:1. Introduction
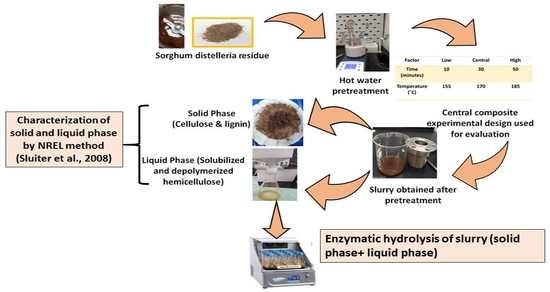
2. Materials and Methods
2.1. Sorghum Distillery Residue Autohydrolysis
2.2. Enzymatic Saccharification
2.3. Analytical Method
3. Results and Discussions
3.1. Chemical Composition of Raw Material
3.2. Operational Variable Effects on Solid Phase Characterization
3.3. Variation in Liquid Phase Composition Corresponding to the Severity
3.4. Pretreatment Slurry Subjected to Enzymatic Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Singhania, R.R.; Soam, S.; Chen, C.; Haldar, D.; Varjani, S.; Chang, J.-S.; Dong, C.-D.; Patel, A.K. Production of Bioethanol from Food Waste: Status and Perspectives. Bioresour. Technol. 2022, 360, 127651. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal Pretreatment Technologies for Lignocellulosic Biomass: A Review of Steam Explosion and Subcritical Water Hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Bertacchi, S.; Jayaprakash, P.; Morrissey, J.P.; Branduardi, P. Interdependence between Lignocellulosic Biomasses, Enzymatic Hydrolysis and Yeast Cell Factories in Biorefineries. Microb. Biotechnol. 2022, 15, 985–995. [Google Scholar] [CrossRef]
- Singh, A.; Rodríguez Jasso, R.M.; Gonzalez-Gloria, K.D.; Rosales, M.; Belmares Cerda, R.; Aguilar, C.N.; Singhania, R.R.; Ruiz, H.A. The Enzyme Biorefinery Platform for Advanced Biofuels Production. Bioresour. Technol. Rep. 2019, 7, 100257. [Google Scholar] [CrossRef]
- Singh, A.; Patel, A.K.; Adsul, M.; Singhania, R.R. Genetic Modification: A Tool for Enhancing Cellulase Secretion. Biofuel Res. J. 2017, 4, 600–601. [Google Scholar] [CrossRef] [Green Version]
- Vaishnav, N.; Singh, A.; Adsul, M.; Dixit, P.; Sandhu, S.K.; Mathur, A.; Puri, S.K.; Singhania, R.R. Penicillium: The next Emerging Champion for Cellulase Production. Bioresour. Technol. Rep. 2018, 2, 131–140. [Google Scholar] [CrossRef]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Sharma, G.D.; Ranjitha, J.; Gupta, V.K. Second-Generation Bioethanol Production from corncob–A Comprehensive Review on Pretreatment and Bioconversion Strategies, Including Techno-Economic and Lifecycle Perspective. Ind. Crops Prod. 2022, 186, 115245. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Lab (NREL): Golden, CO, USA, 2011. [Google Scholar]
- Chen, W.-H.; Lo, H.-J.; Yu, K.-L.; Ong, H.-C.; Sheen, H.-K. Valorization of Sorghum Distillery Residue to Produce Bioethanol for Pollution Mitigation and Circular Economy. Environ. Pollut. 2021, 285, 117196. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Tsai, M.L.; Chen, C.W.; Dong, C.D. Advances and Challenges in Biocatalysts Application for High Solid-Loading of Biomass for 2nd Generation Bio-Ethanol Production. Catalysts 2022, 12, 615. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin valorisation via enzymes: A sustainable approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Singh, A.; Haldar, D.; Soam, S.; Chen, C.-W.; Tsai, M.-L.; Dong, C.-D. Consolidated Bioprocessing of Lignocellulosic Biomass: Technological Advances and Challenges. Bioresour. Technol. 2022, 354, 127153. [Google Scholar] [CrossRef] [PubMed]
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834. [Google Scholar] [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of Pretreatment Techniques on Enzymatic Hydrolysis of Food Waste. Biomass Convers. Biorefinery 2021, 11, 219–226. [Google Scholar] [CrossRef]
- Gao, M.; Zou, H.; Tian, W.; Shi, D.; Chai, H.; Gu, L.; He, Q.; Tang, W.Z. Co-Digestive Performance of Food Waste and Hydrothermal Pretreated Corn Cob. Sci. Total Environ. 2021, 768, 144448. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Cai, C.M.; Xu, F.; Singh, S.K.; Bansal, N.; Phongpreecha, T.; Dutta, T.; Foster, C.E.; Kumar, R.; Simmons, B.A. Performance of Three Delignifying Pretreatments on Hardwoods: Hydrolysis Yields, Comprehensive Mass Balances, and Lignin Properties. Biotechnol. Biofuels 2019, 12, 213. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Tsai, M.-L.; Chen, C.; Singhania, R.R.; Patel, A.K.; Tambat, V.; Dong, C.-D. Role of Hydrothermal Pretreatment towards Sustainable Biorefinery. Bioresour. Technol. 2022, 367, 128271. [Google Scholar] [CrossRef]
- Singh, A.; Jasso, R.M.R.; Saxena, R.; Cerda, R.B.; Singhania, R.R.; Ruiz, H.A. Subcritical Water Pretreatment for Agave Bagasse Fractionation from Tequila Production and Enzymatic Susceptibility. Bioresour. Technol. 2021, 338, 125536. [Google Scholar] [CrossRef]
- Marques, N.P.; deCassia Pereira, J.; Gomes, E.; daSilva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and Xylanases Production by Endophytic Fungi by Solid State Fermentation Using Lignocellulosic Substrates and Enzymatic Saccharification of Pretreated Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Hu, J.; Shen, F.; Huang, M.; Zhao, L.; Zou, J.; Tian, D.; Jiang, Q.; Zeng, Y. Tuning Hydrothermal Pretreatment Severity of Wheat Straw to Match Energy Application Scenarios. Ind. Crops Prod. 2022, 176, 114326. [Google Scholar] [CrossRef]
- Buitrón, G.; Hernández-Juárez, A.; Hernández-Ramírez, M.D.; Sánchez, A. Biochemical Methane Potential from Lignocellulosic Wastes Hydrothermally Pretreated. Ind. Crops Prod. 2019, 139, 111555. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.-N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.-C.; Yang, B. Severity Factor Kinetic Model as a Strategic Parameter of Hydrothermal Processing (Steam Explosion and Liquid Hot Water) for Biomass Fractionation under Biorefinery Concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, D.L.; Rodríguez-Jasso, R.M.; Zanuso, E.; deRodríguez, D.J.; Amaya-Delgado, L.; Sanchez, A.; Ruiz, H.A. Scale-up and Evaluation of Hydrothermal Pretreatment in Isothermal and Non-Isothermal Regimen for Bioethanol Production Using Agave Bagasse. Bioresour. Technol. 2018, 263, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Templeton, D. Determination of Ash in Biomass; Tp-510-42622; Laboratory Analytical Procedure (LAP) NREL: Golden, CO, USA, 2008. [Google Scholar]
- Ruiz, H.A.; Vicente, A.A.; Teixeira, J.A. Kinetic Modeling of Enzymatic Saccharification Using Wheat Straw Pretreated under Autohydrolysis and Organosolv Process. Ind. Crops Prod. 2012, 36, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Comparison of Microwave and Conduction-Convection Heating Autohydrolysis Pretreatment for Bioethanol Production. Bioresour. Technol. 2017, 243, 273–283. [Google Scholar] [CrossRef]
- Dai, L.; Gu, Y.; Xu, J.; Guo, J.; Jiang, K.; Zhou, X.; Xu, Y. Toward Green Production of Xylooligosaccharides and Glucose from Sorghum Straw Biowaste by Sequential Acidic and Enzymatic Hydrolysis. Ind. Crops Prod. 2022, 179, 114662. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, W.-J.; Pang, B.; Sun, Z.; Lam, S.S.; Sonne, C.; Yuan, T.-Q. Ultrastructural Change in Lignocellulosic Biomass during Hydrothermal Pretreatment. Bioresour. Technol. 2021, 341, 125807. [Google Scholar] [CrossRef]
- Ma, X.J.; Cao, S.L.; Lin, L.; Luo, X.L.; Hu, H.C.; Chen, L.H.; Huang, L.L. Hydrothermal Pretreatment of Bamboo and Cellulose Degradation. Bioresour. Technol. 2013, 148, 408–413. [Google Scholar] [CrossRef]
- de Oliveira Gorgulho Silva, C.; de Castro Moreira dos Santos Júnior, A.; Santana, R.H.; Krüger, R.H.; Fontes, W.; de Sousa, M.V.; Ricart, C.A.O.; Filho, E.X.F. Mild Hydrothermal Pretreatment of Sugarcane Bagasse Enhances the Production of Holocellulases by Aspergillus Niger. J. Ind. Microbiol. Biotechnol. 2019, 46, 1517–1529. [Google Scholar] [CrossRef]
- Batista, G.; Souza, R.B.A.; Pratto, B.; dos Santos-Rocha, M.S.R.; Cruz, A.J.G. Effect of Severity Factor on the Hydrothermal Pretreatment of Sugarcane Straw. Bioresour. Technol. 2019, 275, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ambye-Jensen, M.; Balzarotti, R.; Thomsen, S.T.; Fonseca, C.; Kádár, Z. Combined Ensiling and Hydrothermal Processing as Efficient Pretreatment of Sugarcane Bagasse for 2G Bioethanol Production. Biotechnol. Biofuels 2018, 11, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Tian, D.; Hu, J.; Shen, F.; Zeng, Y.; Yang, G.; Huang, C.; Long, L.; Deng, S. Evaluation of Hydrothermal Pretreatment on Lignocellulose-Based Waste Furniture Boards for Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2020, 192, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery Residues from Cistus Ladanifer (Rockrose) as Feedstock for the Production of Added-Value Phenolic Compounds and Hemicellulosic Oligosaccharides. BioEnergy Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Lei, X.; Wang, F.-F.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. One-Pot Catalytic Conversion of Carbohydrate Biomass to Lactic Acid Using an ErCl3 Catalyst. Appl. Catal. Gen. 2014, 482, 78–83. [Google Scholar] [CrossRef]
- Sofia, T.; Costanzo, P.; Sonia, B.; Macario, A.; DiGioia, M.L.; Nardi, M.; Antonio, P.; Oliverio, D.M. Combined Ultrasound/microwave Chemocatalytic Method for Selective Conversion of Cellulose into Lactic Acid. Sci. Rep. 2019, 9, 18858. [Google Scholar]
- Li, H.; Chen, X.; Ren, J.; Deng, H.; Peng, F.; Sun, R. Functional Relationship of Furfural Yields and the Hemicellulose-Derived Sugars in the Hydrolysates from Corncob by Microwave-Assisted Hydrothermal Pretreatment. Biotechnol. Biofuels 2015, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Imman, S.; Laosiripojana, N.; Champreda, V. Effects of Liquid Hot Water Pretreatment on Enzymatic Hydrolysis and Physicochemical Changes of Corncobs. Appl. Biochem. Biotechnol. 2018, 184, 432–443. [Google Scholar] [CrossRef]
- Souto, L.R.F.; daSilva, I.F.; Ninow, J.L.; Collins, S.R.A.; Elliston, A.; Waldron, K.W. Effect of Hydrothermal Pre-Treatment on Duckweed (Landoltia Punctata) Biomass for Simultaneous Saccharification and Fermentation Process. Biomass Bioenergy 2019, 127, 105259. [Google Scholar] [CrossRef]
- Flannelly, T.; Lopes, M.; Kupiainen, L.; Dooley, S.; Leahy, J.J. Non-Stoichiometric Formation of Formic and Levulinic Acids from the Hydrolysis of Biomass Derived Hexose Carbohydrates. RSC Adv. 2016, 6, 5797–5804. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Romaní, A.; Gomes, D.; Cunha, J.T.; Gama, F.M.; Domingues, L. Recombinant Family 3 Carbohydrate-Binding Module as a New Additive for Enhanced Enzymatic Saccharification of Whole Slurry from Autohydrolyzed Eucalyptus Globulus Wood. Cellulose 2018, 25, 2505–2514. [Google Scholar] [CrossRef] [Green Version]
- Balan, R.; Antczak, A.; Brethauer, S.; Zielenkiewicz, T.; Studer, M.H. Steam Explosion Pretreatment of Beechwood. Part 1: Comparison of the Enzymatic Hydrolysis of Washed Solids and Whole Pretreatment Slurry at Different Solid Loadings. Energies 2020, 13, 3653. [Google Scholar] [CrossRef]
- Sun, J.; Konda, N.M.; Parthasarathi, R.; Dutta, T.; Valiev, M.; Xu, F.; Simmons, B.A.; Singh, S. One-Pot Integrated Biofuel Production Using Low-Cost Biocompatible Protic Ionic Liquids. Green Chem. 2017, 19, 3152–3163. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Zheng, X.; Xian, X.; Hu, L.; Tao, S.; Zhang, X.; Liu, Y.; Lin, X. Efficient Short-Time Hydrothermal Depolymerization of Sugarcane Bagasse in One-Pot for Cellulosic Ethanol Production without Solid-Liquid Separation, Water Washing, and Detoxification. Bioresour. Technol. 2021, 339, 125575. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Chen, C.-W.; Patel, A.K.; Saini, J.K.; Dong, C.-D.; Singhania, R.R. Valorization of Pineapple Leaves Waste for the Production of Bioethanol. Bioengineering 2022, 9, 557. [Google Scholar] [CrossRef]





| Temperature (°C) | 155 °C | 170 °C | 185 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 |
| Log (logR0) | 4.16 | 4.26 | 4.36 | 4.29 | 4.35 | 4.40 | 4.33 | 4.42 | 4.48 |
| pH | 3.55 | 3.55 | 3.54 | 3.62 | 3.57 | 3.57 | 3.61 | 3.43 | 3.40 |
| Heating rate (°C/min) | 2.09 | 2.07 | 1.99 | 2.00 | 2.05 | 2.07 | 2.06 | 2.03 | 2.01 |
| Liquid phase (g/L) | |||||||||
| Glucose | 0.0042 ± 0.00 | 1.82 ± 0.00 | 0.129 ± 0.00 | 1.78 ± 0.00 | 1.85 ± 0.07 | 1.42 ± 0.00 | 7.42 ± 0.02 | 5.16 ± 0.00 | 6.57 ± 0.28 |
| Xylose | Nd | 3.17 ± 0.06 | 1.90 ± 0.03 | 1.66 ± 0.01 | 1.68 ± 0.06 | 0.95 ± 0.01 | 3.65 ± 0.02 | 2.43 ± 0.01 | Nd |
| Arabinose | 1.52 ± 0.03 | 0.01 ± 0.01 | 1.71 ± 0.09 | 1.26 ± 0.01 | 0.81 ± 0.01 | 0.56 ± 0.00 | 1.66 ± 0.01 | 2.12 ± 0.01 | 2.12 ± 0.03 |
| XOS | 5.38 ± 0.81 | 4.19 ± 0.69 | 4.32 ± 0.11 | 2.48 ± 0.43 | 4.21 ± 0.58 | 1.24 ± 0.18 | Nd | Nd | Nd |
| GOS | 28.96 ± 1.81 | 38.83 ± 1.72 | 23.21 ± 1.37 | 17.76 ± 1.21 | 16.33 ± 2.53 | 5.91 ± 0.05 | 5.82 ± 0.82 | 4.49 ± 0.32 | 0.93 ± 0.74 |
| Acetic acid | Nd | Nd | 0.87 ± 0.00 | 0.54 ± 0.00 | 0.84 ± 0.00 | 1.02 ± 0.00 | 1.19 ± 0.00 | 2.05 ± 0.00 | 2.04 ± 0.00 |
| Furfural | Nd | Nd | 0.36 ± 0.00 | 1.43 ± 0.01 | 2.24 ± 0.00 | 1.49 ± 0.00 | 7.27 ± 0.03 | 6.98 ± 0.00 | 3.71 ± 0.00 |
| HMF | Nd | Nd | 1.23 ± 0.00 | 8.16 ± 0.03 | 3.54 ± 0.00 | 1.06 ± 0.00 | 8.14 ± 0.02 | 8.02 ± 0.00 | 12.40 ± 0.05 |
| Formic acid | 21 ± 0.063 | 0.154 ± 0.082 | 22.91 ± 0.48 | 17.01 ± 0.00 | 15.92 ± 0.00 | 13.39 ± 0.00 | 7.92 ± 0.00 | 15.66 ± 0.00 | 32.6 ± 0.51 |
| Lactic acid | 6.06 ± 0.01 | 0.05 ± 0.03 | 6.54 ± 0.02 | 4.45 ± 0.05 | 3.71 ± 0.02 | 2.54 ± 0.01 | 9.94 ± 0.05 | 6.65 ± 0.05 | 6.90 ± 0.01 |
| Cellobiose | 0.97 ± 0.00 | 0.82 ± 0.00 | 1.47 ± 0.00 | 0.11 ± 0.00 | 3.72 ± 0.02 | 0.53 ± 0.00 | 2.09 ± 0.03 | 1.16 ± 0.01 | 1.28 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Chen, C.-W.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. Subcritical Water Pretreatment for the Efficient Valorization of Sorghum Distillery Residue for the Biorefinery Platform. Bioengineering 2023, 10, 38. https://doi.org/10.3390/bioengineering10010038
Singh A, Chen C-W, Patel AK, Dong C-D, Singhania RR. Subcritical Water Pretreatment for the Efficient Valorization of Sorghum Distillery Residue for the Biorefinery Platform. Bioengineering. 2023; 10(1):38. https://doi.org/10.3390/bioengineering10010038
Chicago/Turabian StyleSingh, Anusuiya, Chiu-Wen Chen, Anil Kumar Patel, Cheng-Di Dong, and Reeta Rani Singhania. 2023. "Subcritical Water Pretreatment for the Efficient Valorization of Sorghum Distillery Residue for the Biorefinery Platform" Bioengineering 10, no. 1: 38. https://doi.org/10.3390/bioengineering10010038