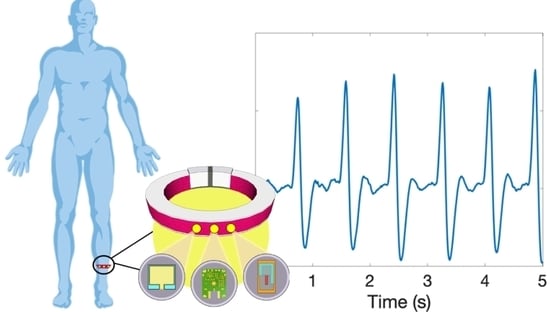
Wearable Heart Rate Monitoring Device Communicating in 5G ISM Band for IoHT
Abstract
:1. Introduction
2. System Design and Characterization
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Windmiller, J.R.; Wang, J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Heimrich, K.G.; Lehmann, T.; Schlattmann, P.; Prell, T. Heart rate variability analyses in Parkinson’s disease: A systematic review and meta-analysis. Brain Sci. 2021, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Kant, N.; Peters, G.M.; Voorthuis, B.J.; Groothuis-Oudshoorn, C.G.; Koning, M.V.; Witteman, B.P.; Rinia-Feenstra, M.; Doggen, C.J. Continuous vital sign monitoring using a wearable patch sensor in obese patients: A validation study in a clinical setting. J. Clin. Monit. Comput. 2022, 36, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Serhani, M.A.; T. El Kassabi, H.; Ismail, H.; Nujum Navaz, A. ECG monitoring systems: Review, architecture, processes, and key challenges. Sensors 2020, 20, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.H.; Dong, L. Flexible Sensors and Machine Learning for Heart Monitoring. Nano Energy 2022, 102, 107632. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. A comprehensive review on the state-of-the-art of piezoelectric energy harvesting. Nano Energy 2021, 80, 105567. [Google Scholar] [CrossRef]
- Demir, S.M.; Al-Turjman, F.; Muhtaroğlu, A. Energy scavenging methods for WBAN applications: A review. IEEE Sens. J. 2018, 18, 6477–6488. [Google Scholar] [CrossRef]
- Natta, L.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Rizzi, F.; Scarpa, E.; Qualtieri, A.; Todaro, M.T.; Sallustio, V.; De Vittorio, M. Conformable AlN Piezoelectric Sensors as a Non-invasive Approach for Swallowing Disorder Assessment. ACS Sens. 2021, 6, 1761–1769. [Google Scholar] [CrossRef]
- Marasco, I.; Niro, G.; Rizzi, F.; Vittorio, M.; D’Orazio, A.; Grande, M. Design of a PEN-Based Flexible PIFA Antenna Operating in the sub-6GHz Band for 5G Applications. In Proceedings of the 2020 22nd International Conference on Transparent Optical Networks (ICTON), Bari, Italy, 19–23 July 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Marasco, I.; Niro, G.; Rizzi, F.; Vittorio, M.; D’Orazio, A.; Grande, M. A compact evolved antenna for 5G communications. Sci. Rep. 2022, 12, 10327. [Google Scholar] [CrossRef]
- Kim, S.; Pakzad, S.; Culler, D.; Demmel, J.; Fenves, G.; Glaser, S.; Turon, M. Health monitoring of civil infrastructures using wireless sensor networks. In Proceedings of the 6th International Conference on Information Processing in Sensor Networks, Cambridge, MA, USA, 25–27 April 2007; pp. 254–263. [Google Scholar] [CrossRef]
- Ketu, S.; Mishra, P.K. Internet of Healthcare Things: A contemporary survey. J. Netw. Comput. Appl. 2021, 192, 103179. [Google Scholar] [CrossRef]
- Gubbi, J.; Buyya, R.; Marusic, S.; Palaniswami, M. Internet of Things (IoT): A vision, architectural elements, and future directions. Future Gener. Comput. Syst. 2013, 29, 1645–1660. [Google Scholar] [CrossRef] [Green Version]
- Niro, G.; Marasco, I.; Lamanna, L.; Rizzi, F.; D’Orazio, A.; de Vittorio, M.; Grande, M. Fabrication of a Flexible Film Bulk Acoustic Resonator for Wireless Sensor Networks. In Proceedings of the 2022 Microwave Mediterranean Symposium (MMS), Pizzo Calabro, Italy, 9–13 May 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Al Ahmad, M.; Ahmed, S. Heart-rate and pressure-rate determination using piezoelectric sensor from the neck. In Proceedings of the 2017 4th IEEE International Conference on Engineering Technologies and Applied Sciences (ICETAS), Salmabad, Bahrain, 29 November–1 December 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, D.G.; Park, J.W.; Youm, S.K. Wearable sensing of in-ear pressure for heart rate monitoring with a piezoelectric sensor. Sensors 2015, 15, 23402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.; Zhang, J.; Shao, Z.; Wang, G.; Geng, X.; Zhang, Y.; Zhang, H. A Wearable and Real-Time Pulse Wave Monitoring System Based on a Flexible Compound Sensor. Biosensors 2022, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, S.; Zhu, R. A wearable multifunctional pulse monitor using thermosensation-based flexible sensors. IEEE Trans. Biomed. Eng. 2018, 66, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Union, I.T. Radio Regulations Articles. ITU Radio Regul. 2012, IV, 227–233. [Google Scholar]
- Christoe, M.J.; Yuan, J.; Michael, A.; Kalantar-Zadeh, K. Bluetooth signal attenuation analysis in human body tissue analogues. IEEE Access 2021, 9, 85144–85150. [Google Scholar] [CrossRef]
- Coviello, G.; Florio, A.; Avitabile, G.; Talarico, C.; Wang-Roveda, J.M. Distributed Full Synchronized System for Global Health Monitoring Based on FLSA. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 600–608. [Google Scholar] [CrossRef]
- Jara, A.J.; Fern’ndez, D.; López, P.; Zamora, M.A.; Ubeda, B.; Skarmeta, A.G. Evaluation of bluetooth low energy capabilities for continuous data transmission from a wearable electrocardiogram. In Proceedings of the 2012 Sixth International Conference on Innovative Mobile and Internet Services in Ubiquitous Computing, Palermo, Italy, 4–6 July 2012; pp. 912–917. [Google Scholar] [CrossRef]
- Bayramzadeh, S.; Aghaei, P. Technology integration in complex healthcare environments: A systematic literature review. Appl. Ergon. 2021, 92, 103351. [Google Scholar] [CrossRef]
- Martin, R.M. Piezoelectricity. Phys. Rev. B 1972, 5, 1607–1613. [Google Scholar] [CrossRef]
- Mazda, F. Telecommunications Engineer’s Reference Book; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Li, P.; Karmakar, C.; Liu, C.; Liu, C. Analysing effect of heart rate and age on radial artery pressure derived systolic and diastolic durations in healthy adults. In Proceedings of the 2015 Computing in Cardiology Conference (CinC), Nice, France, 6–9 September 2015; pp. 381–384. [Google Scholar] [CrossRef]
- Omron Healthcare. M3 Comfort Upper Arm Blood Pressure Monitor; Omron Healthcare: Hoofddorp, The Netherlands, 2010. [Google Scholar]
- Xin, Y.; Qi, X.; Qian, C.; Tian, H.; Ling, Z.; Jiang, Z. A Wearable Respiration and Pulse Monitoring System Based on PVDF Piezoelectric Film. Integr. Ferroelectr. 2014, 158, 43–51. [Google Scholar] [CrossRef]
- Mishra, A.; Li, C. A low power 5.8-GHz ISM-band intermodulation radar system for target motion discrimination. IEEE Sens. J. 2019, 19, 9206–9214. [Google Scholar] [CrossRef]




| Sensor Type | Sensor Location | Comm. Type | Central Freq. (GHz) | ISM Bandwidth (MHz) | Max. Indoor Distance (m) | Tx Power (dBm) | |
|---|---|---|---|---|---|---|---|
| [18] | Thermo-sensation | Wrist | Digital | 2.4 (BLE) | 100 | <100 | 0 |
| [15] | Piezoelectric | Neck | Digital | 0.868 (ZigBee) | 5 | <100 | 20 |
| [28] | Piezoelectric (PVDF) | Wrist | Digital | 2.4 (BT) | 100 | <100 * | 20 * |
| [17] | MEMS Pressure | Wrist | Digital | 2.4 (BT) | 100 | <100 * | 20 * |
| [16] | Piezoelectric | In-ear | Analog | 2.4 | 100 | NA | 0 |
| [29] | RFID Tag | Chest | Analog | 5.8 (5G ISM) | 150 | NA | 35 |
| This Work | Piezoelectric (AlN) | Ankle | Analog | 5.8 (5G ISM) | 150 | 55 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasco, I.; Niro, G.; Demir, S.M.; Marzano, L.; Fachechi, L.; Rizzi, F.; Demarchi, D.; Motto Ros, P.; D’Orazio, A.; Grande, M.; et al. Wearable Heart Rate Monitoring Device Communicating in 5G ISM Band for IoHT. Bioengineering 2023, 10, 113. https://doi.org/10.3390/bioengineering10010113
Marasco I, Niro G, Demir SM, Marzano L, Fachechi L, Rizzi F, Demarchi D, Motto Ros P, D’Orazio A, Grande M, et al. Wearable Heart Rate Monitoring Device Communicating in 5G ISM Band for IoHT. Bioengineering. 2023; 10(1):113. https://doi.org/10.3390/bioengineering10010113
Chicago/Turabian StyleMarasco, Ilaria, Giovanni Niro, Suleyman Mahircan Demir, Lorenzo Marzano, Luca Fachechi, Francesco Rizzi, Danilo Demarchi, Paolo Motto Ros, Antonella D’Orazio, Marco Grande, and et al. 2023. "Wearable Heart Rate Monitoring Device Communicating in 5G ISM Band for IoHT" Bioengineering 10, no. 1: 113. https://doi.org/10.3390/bioengineering10010113