Identification of Antimycobacterial Natural Products from a Library of Marine Invertebrate Extracts
Abstract
:1. Introduction
2. Results and Discussion
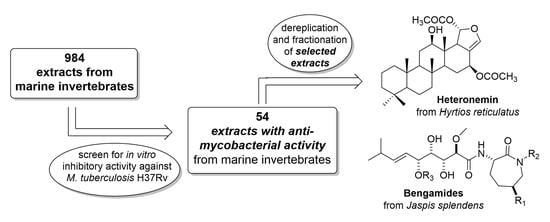
2.1. Screening of Marine Invertebrate Samples
2.2. Dereplication, Molecular Networking, Isolation and Structure Elucidation
2.3. Antimycobacterial Activity of Isolated Compounds and Semi-Pure Fractions
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Marine Invertebrate Samples
3.3. Antimycobacterial Activity
3.4. Fractionation, Isolation and Purification of Active Compounds
3.5. Dereplication and Molecular Networking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Snelll, N.J.C. The treatment of tuberculosis: Current status and future prospects. Expert Opin. Investig. Drugs 1998, 7, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G.A. Current perspectives in drug discovery against tuberculosis from natural products. Int. J. Mycobacteriol. 2015, 4, 165–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, G.; Pasca, M.R. Trends in discovery of new drugs for tuberculosis therapy. J. Antibiot. 2014, 67, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Blanco, D.; Perez-Herran, E.; Cacho, M.; Ballell, L.; Castro, J.; Del Río, R.G.; Lavandera, J.L.; Remuiñán, M.J.; Richards, C.; Rullas, J.; et al. Mycobacterium tuberculosis Gyrase Inhibitors as a New Class of Antitubercular Drugs. Antimicrob. Agents Chemother. 2015, 59, 1868–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, V.J.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2008, 25, 662–695. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2013, 19, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Bowersox, S.S.; Luther, R. Pharmacotherapeutic potential of Omega- Conotoxin MVIIA (SNX-111), an N-type neuronal calcium channel blocker found in the venom of Conus magus. Toxicon 1998, 36, 1651–1658. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar] [CrossRef]
- Hou, X.; Wang, C.; Gerwick, W.H.; Shao, C. European Journal of Medicinal Chemistry Marine natural products as potential anti-tubercular agents. Eur. J. Med. Chem. 2019, 165, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Britt, J.R.; Evans, J.R.; Akee, R.K.; Whitt, J.A.; Trinh, S.K.; Harris, M.J.; Thompsonm, J.R.; Ewing, T.L.; Shipley, S.M.; et al. NCI Program for Natural Product Discovery: A Publicly-Accessible Library of Natural Product Fractions for High-Throughput Screening. ACS Chem. Biol. 2018, 13, 2484–2497. [Google Scholar] [CrossRef] [PubMed]
- Sass, P. Postgenomic strategies in antibacterial drug discovery. Futur. Microbiol 2010, 5, 1553–1579. [Google Scholar]
- White, K.N.; Tenney, K.; Crews, P. The Bengamides: A Mini-Review of Natural Sources, Analogues, Biological Properties, Biosynthetic Origins, and Future Prospects. J. Nat. Prod. 2017, 80, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Garcí, C.; Sarabia, F. Chemistry and Biology of Bengamides and Bengazoles, Bioactive Natural Products from Jaspis Sponges. Mar. Drugs 2014, 12, 1580–1622. [Google Scholar] [CrossRef] [Green Version]
- Quan, D.H.; Nagalingam, G.; Luck, I.; Proschogo, N.; Pillalamarri, V.; Addlagatta, A.; Martinez, E.; Sintchenko, V.; Rutledge, P.J.; Triccas, J.A. Bengamides display potent activity against drug-resistant Mycobacterium tuberculosis. Sci. Rep. 2019, 9, 14396. [Google Scholar] [CrossRef] [Green Version]
- Collins, L.A.; Torrero, M.N.; Franzblau, S.G. Green Fluorescent Protein Reporter Microplate Assay for High-Throughput Screening of Compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1998, 42, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, G.L.; Kumar, A.; Savvi, S.; Hung, A.W.; Wen, S.; Abell, C.; Barry, E.C.; Sherman, D.R.; Boshoff, H.I.; Mizrahi, V. Pathway-selective sensitization of Mycobacterium tuberculosis for target-based whole-cell screening. Chem. Biol. 2013, 19, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Seleghim, M.H.R.; Lira, S.P.; Kossuga, M.H.; Batista, T.; Berlinck, R.G.S.; Hajdu, E.; Muricy, G.; da Rocha, R.M.; do Nascimento, G.G.F.; Silva, M.; et al. Antibiotic, cytotoxic and enzyme inhibitory activity of crude extracts from Brazilian marine invertebrates. Braz. J. Pharmacogn. 2007, 17, 287–318. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Karinel, N.R.A.D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the Caribbean Sea sponge Neopetrosia proxima. Bioorg Med. Chem Lett 2010, 20, 5905–5908. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Identification of the Target Protein of Agelasine D, a Marine Sponge Diterpene Alkaloid, as an Anti-dormant Mycobacterial Substance. Chem. Bio. Chem. 2014, 35145, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mangalindan, G.C.; Talaue, M.T.; Cruz, L.J.; Franzblau, S.G.; Concepcion, G.P.; Adams, L.B.; Richardson, A.D.; Ireland, C.M. Agelasine F from a Philippine Agelas sp. Sponge Exhibits in vitro Antituberculosis Activity. Planta Med. 2000, 66, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. Heteronemin, A new Scalarin type Sesterterpene from the sponge Heteronema erecta. Tetrahedron Lett. 1976, 17, 2631–2634. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Martin, M.T.; Debitus, C.; Guyot, M. 12-epi-Heteronemin: New Sesterterpene From Sponge the marine sponge Hyrtios erecta. Tetrahedron Lett. 1994, 35, 109–110. [Google Scholar] [CrossRef]
- Networking, S.M. Perspective Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar]
- Thale, Z.; Kinder, F.R.; Bair, K.W.; Bontempo, J.; Czuchta, A.M.; Versace, R.W.; Phillips, P.E.; Sanders, M.L.; Wattanasin, S.; Crews, P. Bengamides revisited: New structures and antitumor studies. J. Org. Chem. 2001, 66, 1733–1741. [Google Scholar] [CrossRef]
- Lu, J.; Yuan, X.; Yuan, H.; Wang, W.; Wan, B.; Franzblau, S.G.; Ye, Q. Inhibition of Mycobacterium tuberculosis methionine aminopeptidases by bengamide derivatives. Chem. Med. Chem. 2012, 6, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yuan, X.; Ye, Q. Structural analysis of inhibition of Mycobacterium tuberculosis methionine aminopeptidase by bengamide derivatives. Eur. J. Med. Chem. 2012, 47, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to Convert Raw Mass Spectrometry Data. Curr. Protoc. Bioinform. 2015, 46, 918–920. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef] [Green Version]


| Country | Phylum | Class | Order | Family | Genus | Species | NPID | MIC90 (μg/mL) | MIC99 (μg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| Mauritius | Porifera | Demospongiae | Astrophorida | Ancorinidae | Jaspis | splendens (Dorypleres) | C019765 | 2.4 | 4.5 |
| Porifera | Demospongiae | Dictyoceratida | Thorectidae | Hyrtios | reticulatus | C019725 | 51.0 | 72.0 | |
| South Africa | Porifera | Demospongiae | Poecilosclerida | Guitarridae | Guitarra | fimbriata indica | C018520 | 70.0 | 87.0 |
| Porifera | Demospongiae | Hadromerida | Suberitidae | Aaptos | sp. 1 | C018442 | 71.0 | 83.0 | |
| Porifera | Demospongiae | Verongida | Aplysinellidae | Porphyria | pedunculata | C018530 | 71.0 | 94.0 | |
| Porifera | Demospongiae | Poecilosclerida | Hymedesmiidae | Phorbas | sp. 1 | C018462 | 72.0 | 81.0 | |
| Porifera | Demospongiae | Halichondrida | Heteroxyidae | Higginsia | bidentifera | C018466 | 72.0 | 83.0 | |
| Porifera | Demospongiae | Halichondrida | Heteroxyidae | Higginsia | sp. 1 | C018578 | 72.0 | 84.0 | |
| Cnidaria | Hydrozoa | Leptothecatae | Aglaopheniidae | Lytocarpia | formosa | C018502 | 73.0 | 81.0 | |
| Porifera | Demospongiae | Halichondrida | Halichondriidae | Hymeniacidon | sp. 1 | C018470 | 73.0 | 84.0 | |
| Porifera | Demospongiae | Poecilosclerida | Guitarridae | Guitarra | fimbriata indica | C018458 | 73.0 | 91.0 | |
| Chordata | Ascidiacea | Aplousobranchia | Didemnidae | Didemnum | obscurum | C019906 | 1.6 | 1.7 | |
| Porifera | Demospongiae | Halichondrida | Dictyonellidae | Stylissa | sp. 1, n.sp. | C019904 | 17.0 | 19.4 | |
| Cnidaria | Anthozoa | Alcyonacea | Nephtheidae | Drifa | sp. b (n.sp.) | C018631 | 41.0 | 52.0 | |
| Porifera | Demospongiae | Poecilosclerida | Raspailiidae | Echinodictyum | sp. 1 | C018566 | 25.1 | 42.8 | |
| Mollusca | Polyplacophora | Chitonida | Acanthochitonidae | Acanthochitona | garnoti | C018637 | 0.5 | 0.63 | |
| Porifera | Demospongiae | Poecilosclerida | Isodictyidae | Isodictya | sp. 2, n.sp. | C018626 | 18.8 | 6.25 | |
| Porifera | Demospongiae | Poecilosclerida | Tedaniidae | Hemitedania | sp. 1 | C018538 | 18.7 | 9.3 | |
| Porifera | Demospongiae | Halichondrida | Halichondriidae | Halichondria | sp. 1 | C018460 | 13.8 | 12.2 | |
| Tanzania | Porifera | Demospongiae | Haplosclerida | Petrosiidae | Neopetrosia | tuberosa cf. | C015405 | 2.5 | 3.2 |
| Porifera | Demospongiae | Haplosclerida | Phloeodictyidae | Oceanapia | ramsayi | C015331 | 22.0 | 28.0 | |
| Porifera | Demospongiae | Haplosclerida | Phloeodictyidae | Oceanapia | sp. 3 | C015461 | 35.0 | 42.0 | |
| Porifera | Demospongiae | Poecilosclerida | Chondropsidae | Chondropsis | sp. 1 | C015479 | 58.0 | 64.0 | |
| Chordata | Ascidiacea | Aplousobranchia | Polycitoridae | Eudistoma | giganteum | C015357 | 61.0 | 68.0 | |
| Porifera | Demospongiae | Dictyoceratida | Thorectidae | Aplysinopsis | elegans cf. | C015228 | 2.5 | 2.60 | |
| Mollusca | Gastropoda | Neogastropoda | Cypraeidae | Cypraea | tigris | C015272 | 47.9 | 57.9 | |
| Porifera | Demospongiae | Poecilosclerida | Podospongiidae | Diacarnus | ardoukobae | C015180 | 70.8 | 81.2 | |
| Papua-New Guinea | Chordata | Ascidiacea | Aplousobranchia | Didemnidae | Lissoclinum | badium | C018795 | 2.6 | 3.1 |
| Porifera | Demospongiae | Dictyoceratida | Thorectidae | Fascaplysinopsis | sp. 2 | C018781 | 7.9 | 16.5 | |
| Porifera | Demospongiae | Haplosclerida | Petrosiidae | Petrosia (Strongylophora) | corticata | C018743 | 31.0 | 35.0 | |
| Porifera | Demospongiae | Dictyoceratida | Dysideidae | Dysidea | sp. 17 | C018675 | 32.0 | 36.0 | |
| Porifera | Demospongiae | Haplosclerida | Chalinidae | Haliclona (Gellius) | sp. 4 | C018717 | 36.0 | 57.0 | |
| Porifera | Demospongiae | Dictyoceratida | Dysideidae | Lamellodysidea | herbacea | C018667 | 41.0 | 43.0 | |
| Porifera | Demospongiae | Dictyoceratida | Thorectidae | Carteriospongia | sp. 3 | C018787 | 48.0 | 70.0 | |
| Porifera | Demospongiae | Dictyoceratida | Thorectidae | Phyllospongia | sp. 7 | C018595 | 63.0 | 70.0 | |
| Porifera | Demospongiae | Dictyoceratida | Thorectidae | Aplysinopsis | elegans | C018651 | 65.0 | 71.0 | |
| Porifera | Demospongiae | Poecilosclerida | Mycalidae | Mycale | setosa | C018783 | 72.0 | 81.0 | |
| Porifera | Demospongiae | Dictyoceratida | Spongiidae | Spongia | sp. 5 | C018671 | 72.0 | 82.0 | |
| Chordata | Ascidiacea | Aplousobranchia | Polyclinidae | Synoicum | castellatum | C018601 | 72.0 | 83.0 | |
| Porifera | Demospongiae | Haplosclerida | Petrosiidae | Neopetrosia | tuberosa cf. | C018593 | 72.0 | 83.0 | |
| Porifera | Demospongiae | ‘Lithistid’ | Theonellidae | Theonella | sp. 7 | C018729 | 73.0 | 83.0 | |
| Porifera | Demospongiae | Dictyoceratida | Irciniidae | Ircinia | arbuscula cf. | C018773 | < 0.24 | 0.5 | |
| Porifera | Demospongiae | Hadromerida | Suberitidae | Aaptos | nigra | C018695 | 58.3 | 51.4 | |
| Porifera | Demospongiae | Agelasida | Agelasidae | Agelas | oxeata cf. | C018756 | 0.4 | 0.9 | |
| Porifera | Demospongiae | ‘Lithistid’ | Scleritodermidae | Aciculites | oxtylota | C018740 | 20.8 | 21.9 | |
| Porifera | Demospongiae | ‘Lithistid’ | Scleritodermidae | Microscleroderma | herdmani | C018684 | 23.7 | 30.6 | |
| Porifera | Demospongiae | Haplosclerida | Callyspongiidae | Callyspongia (Euplacella) | elongata cf. | C018714 | 85.2 | 125.0 | |
| Porifera | Demospongiae | Haplosclerida | Niphatidae | Pachychalina | sp. 11 | C018694 | 17.9 | 46.5 | |
| Porifera | Demospongiae | Astrophorida | Thrombidae | Thrombus | sp. 1 | C018716 | 20.8 | 42.5 | |
| Porifera | Demospongiae | Haplosclerida | Niphatidae | Niphates | elegans | C018720 | 23.8 | 91.3 | |
| Palau Islands | Porifera | Demospongiae | Halichondrida | Halichondriidae | Topsentia | cavernosa | C019960 | 71.9 | 78.9 |
| Porifera | Demospongiae | Hadromerida | Suberitidae | Aaptos | nigra | C019928 | 85.2 | 106.0 | |
| Porifera | Demospongiae | Haplosclerida | Chalinidae | Haliclona (Gellius) | sp. 4 | C020034 | 95.9 | 120.0 | |
| Porifera | Demospongiae | Verongida | Aplysinellidae | Porphyria | sp. 1, n.sp. | C019898 | 113.0 | 125.0 | |
| Control | Rifampicin | 0.02 | 0.03 | ||||||
| Compound/Fraction | MIC90 (µg/mL): 7 Days | MIC90 (µg/mL): 14 Days |
|---|---|---|
| 2 | 2.14 | 1.03 |
| 3 | 62.50 | 31.25 |
| F107 | 1.09 | 0.74 |
| F114 | <0.24 | <0.24 |
| F130 | 0.65 | 0.49 |
| Rifampicin | 0.02 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquah, K.S.; Beukes, D.R.; Seldon, R.; Jordaan, A.; Sunassee, S.N.; Warner, D.F.; Gammon, D.W. Identification of Antimycobacterial Natural Products from a Library of Marine Invertebrate Extracts. Medicines 2022, 9, 9. https://doi.org/10.3390/medicines9020009
Acquah KS, Beukes DR, Seldon R, Jordaan A, Sunassee SN, Warner DF, Gammon DW. Identification of Antimycobacterial Natural Products from a Library of Marine Invertebrate Extracts. Medicines. 2022; 9(2):9. https://doi.org/10.3390/medicines9020009
Chicago/Turabian StyleAcquah, Kojo Sekyi, Denzil R. Beukes, Ronnett Seldon, Audrey Jordaan, Suthananda N. Sunassee, Digby F. Warner, and David W. Gammon. 2022. "Identification of Antimycobacterial Natural Products from a Library of Marine Invertebrate Extracts" Medicines 9, no. 2: 9. https://doi.org/10.3390/medicines9020009