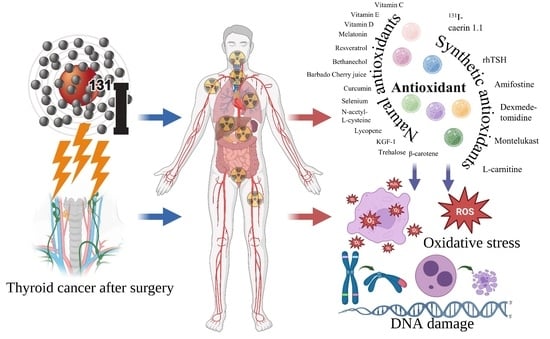
Advances in Antioxidant Applications for Combating 131I Side Effects in Thyroid Cancer Treatment
Abstract
:1. Introduction
2. Side Effects of 131I
2.1. Salivary Gland Dysfunction
2.2. Others
3. Oxidative Stress Dominates 131I Side Effects
4. Antioxidants Reduce 131I Side Effects
4.1. Natural Antioxidant
4.2. Synthetic Antioxidants
4.3. Antioxidant Deficiency
5. Challenges and Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Buczyńska, A.; Sidorkiewicz, I.; Rogucki, M.; Siewko, K.; Adamska, A.; Kościuszko, M.; Maliszewska, K.; Kozłowska, G.; Szumowski, P.; Myśliwiec, J.; et al. Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci. Rep. 2021, 11, 17126. [Google Scholar] [CrossRef]
- Tuttle, R.M. Controversial Issues in Thyroid Cancer Management. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 1187–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, D. Recent Advances in Thyroid Cancer Research. Int. J. Mol. Sci. 2022, 23, 4631. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Shao, C.; Li, Z.; Zhang, C.; Zhang, W.; He, R.; Xu, J.; Cai, Y. Optical diagnostic imaging and therapy for thyroid cancer. Mater. Today Bio 2022, 17, 100441. [Google Scholar] [CrossRef]
- Ma, C.; Xie, J.; Liu, W.; Wang, G.; Zuo, S.; Wang, X.; Wu, F. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst. Rev. 2010, 2010, CD008302. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar]
- Jin, Y.; Ruan, M.; Cheng, L.; Fu, H.; Liu, M.; Sheng, S.; Chen, L. Radioiodine Uptake and Thyroglobulin-Guided Radioiodine Remnant Ablation in Patients with Differentiated Thyroid Cancer: A Prospective, Randomized, Open-Label, Controlled Trial. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Papastavrou, E.; Merkouris, A.; Frangos, S.; Tamana, P.; Charalambous, A. Clinical Studies of Nonpharmacological Methods to Minimize Salivary Gland Damage after Radioiodine Therapy of Differentiated Thyroid Carcinoma: Systematic Review. Evid. Based Complement. Altern. Med. ECAM 2016, 2016, 6795076. [Google Scholar] [CrossRef] [Green Version]
- Monteiro Gil, O.; Oliveira, N.G.; Rodrigues, A.S.; Laires, A.; Ferreira, T.C.; Limbert, E.; Léonard, A.; Gerber, G.; Rueff, J. Cytogenetic alterations and oxidative stress in thyroid cancer patients after iodine-131 therapy. Mutagenesis 2000, 15, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, A.P.; Düsman, E.; Mariucci, R.G.; Lopes, N.B.; Vicentini, V.E.P. Antimutagenic and radioprotective activities of beta-carotene against the biological effects of iodine-131 radiopharmaceutical in Wistar rats. Genet. Mol. Res. GMR 2014, 13, 2248–2258. [Google Scholar] [CrossRef]
- Signore, A.; Campagna, G.; Marinaccio, J.; de Vitis, M.; Lauri, C.; Berardinelli, F.; Tofani, A.; Chianelli, M.; Borro, M.; Gentile, G.; et al. Analysis of Short-Term and Stable DNA Damage in Patients with Differentiated Thyroid Cancer Treated with 131I in Hypothyroidism or with Recombinant Human Thyroid-Stimulating Hormone for Remnant Ablation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Wang, K.; Guo, Y.; Li, H.; Zhu, Z.; Huang, X.; Gong, C.; Gao, Y.; Guo, R.; Yang, B.; et al. Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles. J. Nanobiotechnol. 2022, 20, 203. [Google Scholar] [CrossRef] [PubMed]
- Efanov, A.A.; Brenner, A.V.; Bogdanova, T.I.; Kelly, L.M.; Liu, P.; Little, M.P.; Wald, A.I.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. Investigation of the Relationship Between Radiation Dose and Gene Mutations and Fusions in Post-Chernobyl Thyroid Cancer. JNCI J. Natl. Cancer Inst. 2017, 110, 371–378. [Google Scholar] [CrossRef]
- Ish-Shalom, S.; Durleshter, L.; Segal, E.; Nagler, R.M. Sialochemical and oxidative analyses in radioactive I131-treated patients with thyroid carcinoma. Eur. J. Endocrinol. 2008, 158, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Fard-Esfahani, A.; Emami-Ardekani, A.; Fallahi, B.; Fard-Esfahani, P.; Beiki, D.; Hassanzadeh-Rad, A.; Eftekhari, M. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl. Med. Commun. 2014, 35, 808–817. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Feng, S. The Antioxidant Capacity In Vitro and In Vivo of Polysaccharides From Bergenia emeiensis. Int. J. Mol. Sci. 2020, 21, 7456. [Google Scholar] [CrossRef]
- Carsono, N.; Tumilaar, S.G.; Kurnia, D.; Latipudin, D.; Satari, M.H. A Review of Bioactive Compounds and Antioxidant Activity Properties of Piper Species. Molecules 2022, 27, 6774. [Google Scholar] [CrossRef]
- Skała, E.; Sitarek, P.; Różalski, M.; Krajewska, U.; Szemraj, J.; Wysokińska, H.; Śliwiński, T. Antioxidant and DNA Repair Stimulating Effect of Extracts from Transformed and Normal Roots of Rhaponticum carthamoides against Induced Oxidative Stress and DNA Damage in CHO Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5753139. [Google Scholar] [CrossRef] [Green Version]
- Coskun, M.; Kayis, T.; Gulsu, E.; ALP, E. Effects of Selenium and Vitamin E on Enzymatic, Biochemical, and Immunological Biomarkers in Galleria mellonella L. Sci. Rep. 2020, 10, 9953. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Alavi, M.; Zal, F. The evaluation of protective and mitigating effects of vitamin C against side effects induced by radioiodine therapy. Radiat. Environ. Biophys. 2018, 57, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Jafarpour, S.M.; Mohseni, M.; Salimian, M.; Akbari, H.; Karami, F.; Aliasgharzadeh, A.; Farhood, B. Vitamins E and C Prevent DNA Double-strand Breaks in Peripheral Lymphocytes Exposed to Radiations from Iodine-131. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2018, 33, 20–24. [Google Scholar]
- Almeida, I.V.; Düsman, E.; Heck, M.C.; Pamphile, J.A.; Lopes, N.B.; Tonin, L.T.D.; Vicentini, V.E.P. Cytotoxic and mutagenic effects of iodine-131 and radioprotection of acerola (Malpighia glabra L.) and beta-carotene in vitro. Genet. Mol. Res. GMR 2013, 12, 6402–6413. [Google Scholar] [CrossRef]
- Juweid, M.E.; Tulchinsky, M.; Mismar, A.; Momani, M.; Zayed, A.A.; Al Hawari, H.; Albsoul, N.; Mottaghy, F.M. Contemporary considerations in adjuvant radioiodine treatment of adults with differentiated thyroid cancer. Int. J. Cancer 2020, 147, 2345–2354. [Google Scholar] [CrossRef]
- Estorch, M.; Mitjavila, M.; Muros, M.A.; Caballero, E.; en nombre del Grupo de Trabajo de Endocrinología de la SEMNIM. Radioiodine treatment of differentiated thyroid cancer related to guidelines and scientific literature. Rev. Espanola Med. Nucl. E Imagen Mol. 2019, 38, 195–203. [Google Scholar] [CrossRef]
- Araque, K.A.; Gubbi, S.; Klubo-Gwiezdzinska, J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020, 52, 562–577. [Google Scholar] [CrossRef]
- Choudhury, P.S.; Gupta, M. Differentiated thyroid cancer theranostics: Radioiodine and beyond. Br. J. Radiol. 2018, 91, 20180136. [Google Scholar] [CrossRef]
- Zhou, W.; Brumpton, B.; Kabil, O.; Gudmundsson, J.; Thorleifsson, G.; Weinstock, J.; Zawistowski, M.; Nielsen, J.B.; Chaker, L.; Medici, M.; et al. GWAS of thyroid stimulating hormone highlights pleiotropic effects and inverse association with thyroid cancer. Nat. Commun. 2020, 11, 3981. [Google Scholar] [CrossRef]
- Culver, C.M.; Dworkin, H.J. Radiation Safety Considerations for Post-Iodine-131 Thyroid Cancer Therapy. J. Nucl. Med. 1992, 33, 1402–1405. [Google Scholar]
- Lin, R.; Banafea, O.; Ye, J. I-131 remnant ablation after thyroidectomy induced hepatotoxicity in a case of thyroid cancer. BMC Gastroenterol. 2015, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.; Jangjoo, S.; Monabati, A.; Masoomi Bandari, D.; Namdari, N. An Unusual Case Report: Occurrence of Renal Cell Carcinoma, Basal Cell Carcinoma and Chronic Lymphocytic Leukemia in a Case of Papillary Thyroid Carcinoma Treated with Radioactive Iodine. Iran. J. Med. Sci. 2018, 43, 659–663. [Google Scholar]
- Büyükşimşek, M.; Paydaş, S.; Oğul, A.; Bağır, E.; Ergin, M. Myeloid Neoplasia and Lymphoblastic Lymphoma with Eosinophilia After Radioactive Iodine: A Case Report. Balk. Med. J. 2018, 35, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Torun, N.; Muratli, A.; Serim, B.D.; Ergulen, A.; Altun, G.D. Radioprotective Effects of Amifostine, L-Carnitine and Vitamin E in Preventing Early Salivary Gland Injury due to Radioactive Iodine Treatment. Curr. Med. Imaging Rev. 2019, 15, 395–404. [Google Scholar] [CrossRef]
- Ma, C.; Xie, J.; Chen, Q.; Wang, G.; Zuo, S. Amifostine for salivary glands in high-dose radioactive iodine treated differentiated thyroid cancer. Cochrane Database Syst. Rev. 2009, 2009, CD007956. [Google Scholar] [CrossRef]
- Clement, S.C.; Peeters, R.P.; Ronckers, C.M.; Links, T.P.; van den Heuvel-Eibrink, M.M.; Nieveen van Dijkum, E.J.M.; van Rijn, R.R.; van der Pal, H.J.H.; Neggers, S.J.; Kremer, L.C.M.; et al. Intermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma—A systematic review. Cancer Treat. Rev. 2015, 41, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Caglar, M.; Tuncel, M.; Alpar, R. Scintigraphic evaluation of salivary gland dysfunction in patients with thyroid cancer after radioiodine treatment. Clin. Nucl. Med. 2002, 27, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, D.C.; Kyula, J.N.; Rosenfelder, N.; Chao-Chu, J.; Kramer-Marek, G.; Khan, A.A.; Roulstone, V.; McLaughlin, M.; Melcher, A.A.; Vile, R.G.; et al. Oncolytic vaccinia virus as a vector for therapeutic sodium iodide symporter gene therapy in prostate cancer. Gene Ther. 2016, 23, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Filetti, S.; Bidart, J.M.; Arturi, F.; Caillou, B.; Russo, D.; Schlumberger, M. Sodium/iodide symporter: A key transport system in thyroid cancer cell metabolism. Eur. J. Endocrinol. 1999, 141, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Jonklaas, J. Role of radioactive iodine for adjuvant therapy and treatment of metastases. J. Natl. Compr. Cancer Netw. JNCCN 2007, 5, 631–640. [Google Scholar] [CrossRef]
- Hesselink, E.N.K.; Brouwers, A.H.; de Jong, J.R.; van der Horst-Schrivers, A.N.A.; Coppes, R.P.; Lefrandt, J.D.; Jager, P.L.; Vissink, A.; Links, T.P. Effects of Radioiodine Treatment on Salivary Gland Function in Patients with Differentiated Thyroid Carcinoma: A Prospective Study. J. Nucl. Med. 2016, 57, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Kogai, T.; Brent, G.A. The sodium iodide symporter (NIS): Regulation and approaches to targeting for cancer therapeutics. Pharmacol. Ther. 2012, 135, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auttara-atthakorn, A.; Sungmala, J.; Anothaisintawee, T.; Reutrakul, S.; Sriphrapradang, C. Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: A systematic review of randomized controlled trials. Front. Endocrinol. 2022, 13, 960265. [Google Scholar] [CrossRef] [PubMed]
- Villoria, M.T.; Gutiérrez-Escribano, P.; Alonso-Rodríguez, E.; Ramos, F.; Merino, E.; Campos, A.; Montoya, A.; Kramer, H.; Aragón, L.; Clemente-Blanco, A. PP4 phosphatase cooperates in recombinational DNA repair by enhancing double-strand break end resection. Nucleic Acids Res. 2019, 47, 10706–10727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokat, A.O.; Akbulut, A.; Billur, D.; Koca, G.; Bayram, P.; Kuru, S.; Karasu, S.; Aydogmus, S.; Cakmak, H.; Ozmert, S.; et al. Montelukast attenuates radioactive I131-induced pulmonary damage on rats. Int. J. Radiat. Biol. 2018, 94, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, B.; Adabi, K.; Majidi, M.; Fard-Esfahani, A.; Heshmat, R.; Larijani, B.; Haghpanah, V. Incidence of second primary malignancies during a long-term surveillance of patients with differentiated thyroid carcinoma in relation to radioiodine treatment. Clin. Nucl. Med. 2011, 36, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Shafaghati, N.; Hedayati, M. Genotoxicity induced by iodine-131 in human cultured lymphocytes. Interdiscip. Toxicol. 2013, 6, 74–76. [Google Scholar] [CrossRef]
- Cazarin, J.; Dupuy, C.; Pires de Carvalho, D. Redox Homeostasis in Thyroid Cancer: Implications in Na+/I− Symporter (NIS) Regulation. Int. J. Mol. Sci. 2022, 23, 6129. [Google Scholar] [CrossRef] [PubMed]
- Purtell, K.; Paroder-Belenitsky, M.; Reyna-Neyra, A.; Nicola, J.P.; Koba, W.; Fine, E.; Carrasco, N.; Abbott, G.W. The KCNQ1-KCNE2 K+ channel is required for adequate thyroid I− uptake. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 3252–3259. [Google Scholar] [CrossRef] [Green Version]
- Pesce, L.; Kopp, P. Iodide transport: Implications for health and disease. Int. J. Pediatr. Endocrinol. 2014, 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Hou, Q.; Sun, M.; Sun, J.; Zhang, B. Targeted Isolation of Antioxidant Constituents from Plantago asiatica L. and In Vitro Activity Assay. Molecules 2020, 25, 1825. [Google Scholar] [CrossRef]
- Kang, J.S.; Han, M.H.; Kim, G.-Y.; Kim, C.M.; Kim, B.W.; Hwang, H.J.; Choi, Y.H. Nrf2-Mediated HO-1 Induction Contributes to Antioxidant Capacity of a Schisandrae Fructus Ethanol Extract in C2C12 Myoblasts. Nutrients 2014, 6, 5667–5678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Peng, R.; Jin, L.; Ma, J.; Yang, Q.; Sun, D.; Wu, W. Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications. Pharmaceutics 2021, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef] [Green Version]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, P.; Chen, H.; Ma, J.; Fang, Y.; Qu, L.; Yang, Q.; Peng, B.; Zhang, X.; Jin, L.; Sun, D. Research progress on extraction technology and biomedical function of natural sugar substitutes. Front. Nutr. 2022, 9, 952147. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Guan, Q.-W.; Chen, F.-H.; Xia, Q.-X.; Yin, X.-X.; Zhou, H.-H.; Mao, X.-Y. Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy. Oxid. Med. Cell. Longev. 2020, 2020, 6687185. [Google Scholar] [CrossRef]
- Norberg, L.E.; Lundquist, P.G. An ultrastructural study of salivary gland radiosensitivity after alpha-adrenergic stimulation. Auris. Nasus. Larynx 1988, 15, 1–17. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, J.; Lei, P.; Wang, L.; Qu, J.; Zhao, J.; Liu, F.; Yan, X.; Wu, W.; Jin, L.; et al. Konjac Glucomannan: An Emerging Specialty Medical Food to Aid in the Treatment of Type 2 Diabetes Mellitus. Foods 2023, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, P.; Sheng, L.; Sun, W.; Zhang, H. Ferroptosis-related gene signature predicts the prognosis of papillary thyroid carcinoma. Cancer Cell Int. 2021, 21, 669. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, T.; Huo, X.; Wang, H.; Wang, J.; Xue, L. Glutamine Transporter SLC1A5 Regulates Ionizing Radiation-Derived Oxidative Damage and Ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 3403009. [Google Scholar] [CrossRef]
- Lin, R.; Fogarty, C.E.; Ma, B.; Li, H.; Ni, G.; Liu, X.; Yuan, J.; Wang, T. Identification of ferroptosis genes in immune infiltration and prognosis in thyroid papillary carcinoma using network analysis. BMC Genom. 2021, 22, 576. [Google Scholar] [CrossRef]
- Kaur, V.; Goyal, A.K.; Ghosh, G.; Chandra Si, S.; Rath, G. Development and characterization of pellets for targeted delivery of 5-fluorouracil and phytic acid for treatment of colon cancer in Wistar rat. Heliyon 2020, 6, e03125. [Google Scholar] [CrossRef] [Green Version]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Díaz-Cubilla, M.; Letón, P.; Luna-Vázquez, C.; Marrón-Romera, M.; Boltes, K. Effect of Carbamazepine, Ibuprofen, Triclosan and Sulfamethoxazole on Anaerobic Bioreactor Performance: Combining Cell Damage, Ecotoxicity and Chemical Information. Toxics 2022, 10, 42. [Google Scholar] [CrossRef]
- Ma, J.; Yong, L.; Lei, P.; Li, H.; Fang, Y.; Wang, L.; Chen, H.; Zhou, Q.; Wu, W.; Jin, L.; et al. Advances in microRNA from adipose-derived mesenchymal stem cell-derived exosome: Focusing on wound healing. J. Mater. Chem. B 2022, 10, 9565–9577. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, P.; Chen, H.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Jin, L.; Sun, D. Advances in lncRNAs from stem cell-derived exosome for the treatment of cardiovascular diseases. Front. Pharmacol. 2022, 13, 986683. [Google Scholar] [CrossRef]
- Chen, H.; Lei, P.; Ji, H.; Yang, Q.; Peng, B.; Ma, J.; Fang, Y.; Qu, L.; Li, H.; Wu, W.; et al. Advances in Escherichia coli Nissle 1917 as a customizable drug delivery system for disease treatment and diagnosis strategies. Mater. Today Bio 2023, 18, 100543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, J.; Wu, W.; Fang, Y.; Liu, F.; Yang, Q.; Hu, X.; Gu, X.; He, Z.; Sun, D.; et al. Effect of aerobic exercise as a treatment on type 2 diabetes mellitus with depression-like behavior zebrafish. Life Sci. 2022, 300, 120578. [Google Scholar] [CrossRef] [PubMed]
- El-Benhawy, S.A.; Fahmy, E.I.; Mahdy, S.M.; Khedr, G.H.; Sarhan, A.S.; Nafady, M.H.; Yousef Selim, Y.A.; Salem, T.M.; Abu-Samra, N.; El Khadry, H.A. Assessment of thyroid gland hormones and ultrasonographic abnormalities in medical staff occupationally exposed to ionizing radiation. BMC Endocr. Disord. 2022, 22, 287. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Konukoğlu, D.; Hatemi, H.H.; Arikan, S.; Demir, M.; Akçay, T. Radioiodine treatment and oxidative stress in thyroidectomised patients for differentiated thyroid cancers. Pharmacol. Res. 1998, 38, 311–315. [Google Scholar] [CrossRef]
- Baugnet-Mahieu, L.; Lemaire, M.; Léonard, E.D.; Léonard, A.; Gerber, G.B. Chromosome aberrations after treatment with radioactive iodine for thyroid cancer. Radiat. Res. 1994, 140, 429–431. [Google Scholar] [CrossRef]
- Ameziane-El-Hassani, R.; Talbot, M.; de Souza Dos Santos, M.C.; Al Ghuzlan, A.; Hartl, D.; Bidart, J.-M.; De Deken, X.; Miot, F.; Diallo, I.; de Vathaire, F.; et al. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, 5051–5056. [Google Scholar] [CrossRef] [Green Version]
- Ballardin, M.; Gemignani, F.; Bodei, L.; Mariani, G.; Ferdeghini, M.; Rossi, A.M.; Migliore, L.; Barale, R. Formation of micronuclei and of clastogenic factor(s) in patients receiving therapeutic doses of iodine-131. Mutat. Res. 2002, 514, 77–85. [Google Scholar] [CrossRef]
- Watanabe, N.; Kanegane, H.; Kinuya, S.; Shuke, N.; Yokoyama, K.; Kato, H.; Tomizawa, G.; Shimizu, M.; Funada, H.; Seto, H. The radiotoxicity of 131I therapy of thyroid cancer: Assessment by micronucleus assay of B lymphocytes. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2004, 45, 608–611. [Google Scholar]
- Bartoc, R.; Dumitrescu, C.; Belgun, M.; Olinescu, R. Oxidative and antioxidative factors in the serum of thyroid cancer patients treated with 131I. Rom. J. Endocrinol. 1993, 31, 85–87. [Google Scholar] [PubMed]
- Livingston, G.K.; Foster, A.E.; Elson, H.R. Effect of in vivo exposure to iodine-131 on the frequency and persistence of micronuclei in human lymphocytes. J. Toxicol. Environ. Health 1993, 40, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.J.; Surrallés, J.; Galofré, P.; Creus, A.; Marcos, R. Radioactive iodine induces clastogenic and age-dependent aneugenic effects in lymphocytes of thyroid cancer patients as revealed by interphase FISH. Mutagenesis 1997, 12, 449–455. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Légal, J.D.; Schlumberger, M.; Aubert, B.; Beron-Gaillard, N.; Gaussen, A.; Parmentier, C. Sequential biological dosimetry after a single treatment with iodine-131 for differentiated thyroid carcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1997, 38, 377–380. [Google Scholar]
- Gundy, S.; Katz, N.; Füzy, M.; Esik, O. Cytogenetic study of radiation burden in thyroid disease patients treated with external irradiation or radioiodine. Mutat. Res. 1996, 360, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, S.M.; Safaei, M.; Mohseni, M.; Salimian, M.; Aliasgharzadeh, A.; Farhood, B. The Radioprotective Effects of Curcumin and Trehalose Against Genetic Damage Caused By I-131. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2018, 33, 99–104. [Google Scholar] [CrossRef]
- Kyrilli, A.; Gacquer, D.; Detours, V.; Lefort, A.; Libert, F.; Twyffels, L.; Van Den Eeckhaute, L.; Strickaert, A.; Maenhaut, C.; De Deken, X.; et al. Dissecting the Role of Thyrotropin in the DNA Damage Response in Human Thyrocytes after 131I, γ Radiation and H2O2. J. Clin. Endocrinol. Metab. 2020, 105, dgz185. [Google Scholar] [CrossRef]
- Rosário, P.W.; Batista, K.C.S.; Calsolari, M.R. Radioiodine-induced oxidative stress in patients with differentiated thyroid carcinoma and effect of supplementation with vitamins C and E and selenium (antioxidants). Arch. Endocrinol. Metab. 2016, 60, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Upadhyaya, A.; Zhou, P.; Meng, Z.; Wang, P.; Zhang, G.; Jia, Q.; Tan, J.; Li, X.; Hu, T.; Liu, N.; et al. Radioprotective effect of vitamin E on salivary glands after radioiodine therapy for differentiated thyroid cancer: A randomized-controlled trial. Nucl. Med. Commun. 2017, 38, 891–903. [Google Scholar] [CrossRef]
- Eksioglu, U.; Atilgan, H.I.; Yakin, M.; Yazihan, N.; Altiparmak, U.E.; Yumusak, N.; Korkmaz, M.; Demir, A.; Ornek, F.; Aribal Ayral, P.; et al. Antioxidant Effects of Vitamin D on Lacrimal Glands against High Dose Radioiodine-Associated Damage in an Animal Model. Cutan. Ocul. Toxicol. 2019, 38, 18–24. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, M.E.; Kim, S.-K.; Kim, J.W.; Kim, Y.-M.; Choi, J.-S. Keratinocyte Growth Factor-1 Protects Radioiodine-Induced Salivary Gland Dysfunction in Mice. Int. J. Environ Res. Public Health 2020, 17, 6322. [Google Scholar] [CrossRef]
- Jafarpour, S.M.; Shekarchi, B.; Bagheri, H.; Farhood, B. The Radioprotective Effects of Melatonin and Nanoselenium on DNA Double-Strand Breaks in Peripheral Lymphocytes Caused by I-131. Indian J. Nucl. Med. 2021, 36, 134–139. [Google Scholar] [CrossRef]
- Düsman, E.; Berti, A.P.; Mariucci, R.G.; Lopes, N.B.; Tonin, L.T.D.; Vicentini, V.E.P. Radioprotective effect of the Barbados Cherry (Malpighia glabra L.) against radiopharmaceutical iodine-131 in Wistar rats in vivo. BMC Complement. Altern. Med. 2014, 14, 41. [Google Scholar] [CrossRef] [Green Version]
- Kurashige, T.; Shimamura, M.; Nagayama, Y. N-Acetyl-L-cysteine protects thyroid cells against DNA damage induced by external and internal irradiation. Radiat. Environ. Biophys. 2017, 56, 405–412. [Google Scholar] [CrossRef]
- Koca, G.; Singar, E.; Akbulut, A.; Yazihan, N.; Yumuşak, N.; Demir, A.; Korkmaz, M. The Effect of Resveratrol on Radioiodine Therapy-Associated Lacrimal Gland Damage. Curr. Eye Res. 2021, 46, 398–407. [Google Scholar] [CrossRef]
- Sadic, M.; Aydinbelge, F.N.; Yumusak, N.; Karakok, E.; Akbulut, A.; Koca, G.; Korkmaz, M. Radioprotective effect of lycopene on the gastrointestinal tract after high-dose radioiodine administration in rat models. Nucl. Med. Commun. 2017, 38, 1041–1046. [Google Scholar] [CrossRef]
- Bohuslavizki, K.H.; Brenner, W.; Klutmann, S.; Hübner, R.H.; Lassmann, S.; Feyerabend, B.; Lüttges, J.; Tinnemeyer, S.; Clausen, M.; Henze, E. Radioprotection of Salivary Glands by Amifostine in High-Dose Radioiodine Therapy. J. Nucl. Med. 1998, 39, 1237–1242. [Google Scholar]
- Lin, R.; Ma, B.; Liu, N.; Zhang, L.; He, T.; Liu, X.; Chen, T.; Liu, W.; Liang, Y.; Wang, T.; et al. Targeted radioimmunotherapy with the iodine-131-labeled caerin 1.1 peptide for human anaplastic thyroid cancer in nude mice. Ann. Nucl. Med. 2021, 35, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Kismet, K.; Sadic, M.; Bag, Y.M.; Atilgan, H.I.; Koca, G.; Onalan, A.K.; Senes, M.; Peker, S.A.; Yumusak, N.; Korkmaz, M. Hepatoprotective Effect of Dexmedetomidine Against Radioiodine Toxicity in Rats: Evaluation of Oxidative Status and Histopathological Changes. Int. Surg. 2016, 101, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çakmak, Y.S.; Aktumsek, A.; Duran, A. Studies on antioxidant activity, volatile compound and fatty acid composition of different parts of Glycyrrhiza echinata L. EXCLI J. 2012, 11, 178–187. [Google Scholar]
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-dependent Anti-tumorigenic Effect of Anti-oxidants In Vivo. Cancer Cell 2007, 12, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Amara, F.; Berbenni, M.; Fragni, M.; Leoni, G.; Viggiani, S.; Ippolito, V.M.; Larocca, M.; Rossano, R.; Alberghina, L.; Riccio, P.; et al. Neuroprotection by Cocktails of Dietary Antioxidants under Conditions of Nerve Growth Factor Deprivation. Oxid. Med. Cell. Longev. 2015, 2015, 217258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.-G.; Lee, K.H.; Chang, J.Y.; Kim, J.-C.; Park, H.W. Production, Characterization, and Antioxidant Activities of an Exopolysaccharide Extracted from Spent Media Wastewater after Leuconostoc mesenteroides WiKim32 Fermentation. ACS Omega 2021, 6, 8171. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Patananan, A.N.; Budenholzer, L.M.; Pedraza, M.E.; Torres, E.R.; Adler, L.N.; Clarke, S.G. The invertebrate Caenorhabditis elegans biosynthesizes ascorbate. Arch. Biochem. Biophys. 2015, 569, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Aydoğan, F.; Atılgan, H.I.; Koca, G.; Yumuşak, N.; Aydın, E.; Sadıç, M.; Korkmaz, M.; Tuncal, S.; Samim, E.E. An evaluation of the radioprotective effect of vitamin E on the salivary glands of radioactive iodine in rats. Kulak Burun Bogaz Ihtis. Derg. 2014, 24, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, R.M.; Budinsky, A.C.; Palumbo, B.; Palumbo, R.; Sinzinger, H. Radioiodine therapy induces dose-dependent in vivo oxidation injury: Evidence by increased isoprostane 8-epi-PGF(2 alpha). J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2002, 43, 1254–1258. [Google Scholar]
- Song, F.-L.; Gan, R.-Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.-B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [Green Version]
- Valdes, F.; Brown, N.; Morales-Bayuelo, A.; Prent-Peñaloza, L.; Gutierrez, M. Adenosine Derivates as Antioxidant Agents: Synthesis, Characterization, in Vitro Activity, and Theoretical Insights. Antioxidants 2019, 8, 468. [Google Scholar] [CrossRef] [Green Version]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Jameel, S.; Hameed, A.; Shah, T.M. Biochemical Profiling for Antioxidant and Therapeutic Potential of Pakistani Chickpea (Cicer arietinum L.) Genetic Resource. Front. Plant Sci. 2021, 12, 663623. [Google Scholar] [CrossRef]
- Gledovic, A.; Janosevic-Lezaic, A.; Tamburic, S.; Savic, S. Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability. Antioxidants 2022, 11, 1898. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Ma, C.; Xie, J.; Jiang, Z.; Wang, G.; Zuo, S. Does Amifostine Have Radioprotective Effects on Salivary Glands in High-Dose Radioactive Iodine-Treated Differentiated Thyroid Cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1778–1785. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Ma, Y. Montelukast attenuates interleukin IL-1β-induced oxidative stress and apoptosis in chondrocytes by inhibiting CYSLTR1 (Cysteinyl Leukotriene Receptor 1) and activating KLF2 (Kruppel Like Factor 2). Bioengineered 2021, 12, 8476–8484. [Google Scholar] [CrossRef]
- Chen, H.; Qian, N.; Yan, L.; Jiang, H. Role of serum vitamin A and E in pregnancy. Exp. Ther. Med. 2018, 16, 5185–5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Zhou, J.; Shen, T.; Wang, X. Target-Guided Isolation of Three Main Antioxidants from Mahonia bealei (Fort.) Carr. Leaves Using HSCCC. Molecules 2019, 24, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Ko, S.-C.; Moon, S.-H.; Lee, S.-H. Protective Effects of Novel Antioxidant Peptide Purified from Alcalase Hydrolysate of Velvet Antler Against Oxidative Stress in Chang Liver Cells In Vitro and in a Zebrafish Model In Vivo. Int. J. Mol. Sci. 2019, 20, 5187. [Google Scholar] [CrossRef] [Green Version]
- Sharafi, S.M.; Rasooli, I.; Owlia, P.; Taghizadeh, M.; Astaneh, S.D.A. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn. Mag. 2010, 6, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Lan, H.-R.; Wu, Z.-Q.; Zhang, L.-H.; Jin, K.-T.; Wang, S.-B. Nanotechnology Assisted Chemotherapy for Targeted Cancer Treatment: Recent Advances and Clinical Perspectives. Curr. Top. Med. Chem. 2020, 20, 2442–2458. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-K.; Gan, X.-X.; Deng, X.-Y.; Shen, F.; Feng, J.-H.; Cai, W.-S.; Liu, Q.-Y.; Miao, J.-H.; Zheng, B.-X.; Xu, B. Potential five-mRNA signature model for the prediction of prognosis in patients with papillary thyroid carcinoma. Oncol. Lett. 2020, 20, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Nanoparticles: Promising Auxiliary Agents for Diagnosis and Therapy of Thyroid Cancers. Cancers 2021, 13, 4063. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Lang, J.; Tan, Z.; Feng, Q.; Zhu, F.; Liu, G.; Ying, Z.; Yu, X.; Feng, H.; et al. Lipid-Peptide-mRNA Nanoparticles Augment Radioiodine Uptake in Anaplastic Thyroid Cancer. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2204334. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Jiang, Z.; Li, L.; Huang, Z. Selenium nanoparticles coated with pH responsive silk fibroin complex for fingolimod release and enhanced targeting in thyroid cancer. Artif. Cells Nanomed. Biotechnol. 2021, 49, 83–95. [Google Scholar] [CrossRef]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Wang, X.-Y.; Zhou, X.-R.; Zhang, C.; Liu, K.-J.; Zhang, F.-Y.; Xiang, B. Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland. Antioxidants 2022, 11, 1414. [Google Scholar] [CrossRef]
- Charalambous, A. Seeking Optimal Management for Radioactive Iodine Therapy-induced Adverse Effects. Asia-Pac. J. Oncol. Nurs. 2017, 4, 319–322. [Google Scholar] [CrossRef]





| Subject | Dose of 131I | Test Site | Side Effects of 131I | Ref. |
|---|---|---|---|---|
| Thirty-one patients in hypothyroidism (HYPO group) and 31 patients in euthyroidism (rhTSH group) | 1850 MBq | blood | In the HYPO patients, the radiation exposure rate, chromosome breaks, SSBs, DSBs, total exchanges (DNA-1), transient unstable DNA damage, stable DNA damage, anti-reactive oxygen metabolites (Anti-ROMs), “FAST” antioxidants (Anti-ROMsF), polymorphisms, DNA mutation score↑ and d-ROMs, “SLOW” antioxidants (Anti-ROMsS)↓ at one week. d-ROMs and Anti-ROMsS↑ at 3 months compared to one week. | [13] |
| Nineteen patients (16 women and three men) suffering from thyroid cancer | 2590 MBq | blood | MN and CA↑ and the in-serum uric acid concentration↑ after 1 month. Thiobarbituric acid-reactive products↓ after 6 months. | [11] |
| Eleven patients already submitted to total thyroidectomy | Between 2.96 and 5.50 GBq | peripheral blood lymphocytes | MN and clastogenic factor↑ | [77] |
| Twenty-two DTC patients | 3.7 GBq | Peripheral blood lymphocytes | MN↑ | [78] |
| Ten patients suffering from thyroid cancer | 1850 MBq | Circulating blood lymphocytes | [75] | |
| A 34 year old male patient | 1780 MBq | Lymphocytes | [80] | |
| Twelve women with papillary or folhcular thyroid cancer | 3700–5500 MBq | Blood lymphocytes | Clastogenic effects, X chromosome-independent aneugenic activity↑ at 1 week after treatment. | [81] |
| Fifty DTC patients | 3.7 GBq | Peripheral lymphocytes | CA↑ approx. 10 days after treatment | [82] |
| Nineteen DTC patients | 1734–2600 MBq | Blood lymphocytes | CA↑ | [84] |
| Drug Type | Drug Treatment | Subject | Dose of 131I | Side Effects of 131I | Drug Efficacy | Ref. |
|---|---|---|---|---|---|---|
| Natural antioxidant | Daily supplementation consisting of 2000 mg vitamin C and 1000 mg vitamin E and 400 µg selenium for 21 days before 131I | Forty patients with thyroid cancer submitted for thyroidectomy (n = 20) | 3.7 GBq | 8-epi-PGF2α↑ | 8-epi-PGF2α↓ | [86] |
| 1500 mg vitamin C daily 2 days after (group 2), 2 days before to 2 days after (group 3), and 2 days before RAI (group 4) | Fifty-eight DTC patients ablated with 131I | 5550 MBq | MDA, CAT↑; GSH↓ | MDA↓ (group 2,3,4); GSH↑ (group 3,4); CAT↓ (group 3,4) | [22] | |
| Groups A, B, and C received vitamin E 100, 200, and 300 mg/day orally, respectively, for a duration of 1 week before to 4 weeks after I therapy | Eighty-two DTC patients with 131I | 100 mCi | UF, UI, EF, and ER↓ | UI, EF, UF, ER↑ | [87] | |
| Vitamin D (200 ng/kg/day) | Wistar albino rats (n = 12) | 111 MBq/kg | TOS, TNF-α, IL-6↑; IL-10, TAS↓ | TOS, TNF-α, IL-6↓; IL-10, TAS ↑ | [88] | |
| Vitamin E (800 IU/day for one week before and four weeks after RAI therapy) | Thirty-six DTC patients with RAI (n = 18) | 3700–5550 MBq | FUR, MUR, MSP, and EF↓ | FUR, MUR, MSP, and EF↑ | [43] | |
| Bethanechol (2 mg orally twice a day) for one month after 131I | Fifty DTC patients with RAI (n = 25) | 97.2 to 213.4 mCi | MUR, MSP, ΔMS, EF↓ | Serum amylase↓ | ||
| Selenium 300 mcg orally for ten days (from three days before until six days after RAI therapy) | Sixteen DTC patients with RAI (n = 8) | 3.7 GBq | Xerostomia, sialadenitis symptoms↑ | Xerostomia, sialadenitis symptoms↓ | ||
| KGF-1 (100 ug/1 mL PBS) | Eighteen C57BL/six mice (n = 6) | 0.01 mCi/g | HIF-1α↑; mucin stained acini, amylase↓; periductal fibrosis↑ | HIF-1α↓; mucin stained acini, amylase↑; periductal fibrosis↓ | [89] | |
| 50 μg curcumin per mL of blood and 5.738 mg trehalose per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 42% (curcumin) and 38% (trehalose) | [84] | |
| 0.0167 mg melatonin per mL of blood and 0.025 mg Se NPs per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 38% (melatonin) and 30% (selenium nanoparticles) | [90] | |
| 0.0666 mg vitamin E per mL of blood and 0.0167 mg vitamin C per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 21.5% (vitamin E) and 36.4% (vitamin C) | [23] | |
| Barbados Cherry juice (5 mg)/100 g | Wistar rats (n = 6) | 25 μCi/100 g | 1,1-diphenyl-2-picrylhydrazyl↑; chromosomal and cellular aberrations↑ | 1,1-diphenyl-2-picrylhydrazyl↑; chromosomal and cellular aberrations↑ | [91] | |
| 20 mmol N-acetyl-L-cysteine | Normal differentiated rat thyroid cell line PCCL3 | 10 μCi/mL | ROS, DBS, MN↑ | ROS, DBS, MN↓ | [92] | |
| 8 mg β-carotene/mL corn oil (0.2 mL/100 g) | Wistar rats (n = 6) | 25 μCi /100 g body weight | CA, MN, water consumption↑ | CA, MN, water consumption↓ | [12] | |
| 20 mg/kg/day resveratrol | Thirty Wistar albino rats (n = 10) | 3 mCi/kg | Caspase-3, TUNEL, TNF-α, IL-6, nuclear factor-kappa-B (NF-кB), TOS↑; IL-10, TAS↓ | Caspase-3, TUNEL, TNF-α, IL-6, NF-кB, TOS↑; TAS↓ | [93] | |
| 1 mL lycopene (5 mg/kg body weight) | Twenty Wistar albino rats (n = 10) | 3 mCi | Duodenal and ileal lamina propria edema, duodenal ulcer, gastric mucosal erosion, and gastric and colon mucosal degeneration↑ | Duodenal and ileal lamina propria edema, duodenal ulcer, gastric mucosal erosion, gastric and colon mucosal degeneration↓ | [94] | |
| Synthetic antioxidants | 200 mg/kg amifostine or L-carnitine | Forty adult guinea pigs | 555–660 MBq | Body weight and thyroid hormone↓ | Body weight and thyroid hormone↑ | [34] |
| 200 mg/kg amifostine to three rabbits/500 mg/m2 amifostine before 131I to eight patients | Five rabbits/17 patients | 1 GBq to rabbits/6 GBq to patients | Reduced parenchymal function in parotid and submandibular glands; xerostomia; lipomatosis | None of the parenchymal function in parotid and submandibular glands reduce, xerostomia and lipomatosis occurred | [95] | |
| rhTSH (1 mg/2 d and 1 mg/1 d before 131I) | Sixty-two patients prepared with rhTSH or by thyroid hormone withdrawal | 1850 MBq | CA, MN, ROS↑ | CA, MN, ROS↓ | [13] | |
| 8 μg of F1 peptide labeled with 200 μCi 131I every 3 days for a total of three times | Nude mice with human anaplastic thyroid cancer | 200 μCi | Weight loss and 131I enter the internal circulation | Constant weight | [96] | |
| Dexmedetomidine (3 μg/kg) | Thirty-six Wistar albino female rats (n = 12) | 111 MBq | MDA, advanced oxidized protein products↑, total sulfur group, CAT↓ | MDA, advanced oxidized protein products↓; total sulfur group, CAT↑; liver protection | [97] | |
| Montelukast (10 mg/kg/day) | Fifty female Wistar albino rats (n = 10) | 111 MBq/kg | Inflammation and pulmonary fibrosis | Reduced the degree of inflammation and pulmonary fibrosis | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Ma, J.; Lei, P.; Yi, J.; Ma, Y.; Huang, Z.; Wang, T.; Ping, H.; Ruan, D.; Sun, D.; et al. Advances in Antioxidant Applications for Combating 131I Side Effects in Thyroid Cancer Treatment. Toxics 2023, 11, 529. https://doi.org/10.3390/toxics11060529
Yang L, Ma J, Lei P, Yi J, Ma Y, Huang Z, Wang T, Ping H, Ruan D, Sun D, et al. Advances in Antioxidant Applications for Combating 131I Side Effects in Thyroid Cancer Treatment. Toxics. 2023; 11(6):529. https://doi.org/10.3390/toxics11060529
Chicago/Turabian StyleYang, Li, Jiahui Ma, Pengyu Lei, Jia Yi, Yilei Ma, Zhongke Huang, Tingjue Wang, Haiyan Ping, Danping Ruan, Da Sun, and et al. 2023. "Advances in Antioxidant Applications for Combating 131I Side Effects in Thyroid Cancer Treatment" Toxics 11, no. 6: 529. https://doi.org/10.3390/toxics11060529