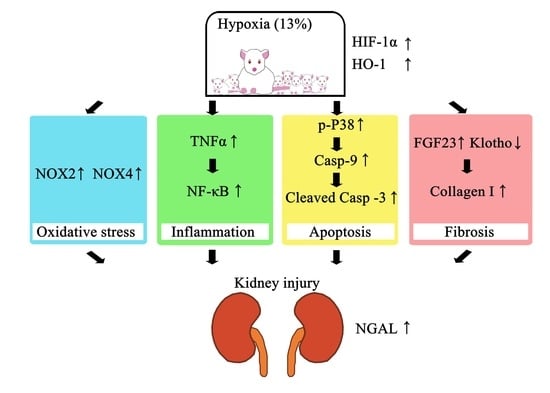
Hypoxia-Induced Kidney Injury in Newborn Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Arterial Blood Gas Measurements
2.3. Tissue Preparation for Histological Studies
2.4. Morphology of the Kidney and Quantitative Assessment of Tubular Injury
2.5. Collagen Fibers and Collagen Volume Fraction (CVF) Detection
2.6. Immunohistochemical Analysis
2.7. Protein Extraction and Determination
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Induction of Hypoxia in Neonatal Rats and Hypoxia Animal Model Establishment
3.2. Blood Gas Analysis
3.3. Effect of Neonatal Hypoxia on Kidney Tissue
3.4. Effects of Neonatal Hypoxia on Body Weights and Protein Expression in the Kidney
3.5. Effect of Neonatal Hypoxia on Fibrotic Change in the Kidney Tissue
3.6. Effects of Neonatal Hypoxia on Oxidative Stress, Apoptosis, and Inflammation in the Kidney
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farahani, R.; Kanaan, A.; Gavrialov, O.; Brunnert, S.; Douglas, R.M.; Morcillo, P.; Haddad, G.G. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr. Pulmonol. 2008, 43, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mach, M.; Dubovicky, M.; Navarova, J.; Brucknerova, I.; Ujhazy, E. Experimental modeling of hypoxia in pregnancy and early postnatal life. Interdiscip. Toxicol. 2009, 2, 28–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulier, V.; Dalmaz, Y.; Cottet-Emard, J.M.; Lagercrantz, H.; Pequignot, J.M. Long-term influence of neonatal hypoxia on catecholamine activity in carotid bodies and brainstem cell groups of the rat. J. Physiol. 1997, 498 Pt 2, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortola, J.P.; Xu, L.; Lauzon, A.-M. Body growth, lung and heart weight, and DNA content in newborn rats exposed to different levels of chronic hypoxia. Can. J. Physiol. Pharmacol. 1990, 68, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Moromisato, D.Y.; Moromisato, M.Y.; Zanconato, S.; Roberts, C.T., Jr. Effect of hypoxia on lung, heart, and liver insulin-like growth factor-I gene and receptor expression in the newborn rat. Crit. Care Med. 1996, 24, 919–924. [Google Scholar] [CrossRef]
- Nabil Azar, M.N.; El Sabban, M.; Bitar, H.; Obeid, M.; Dbaibo, G.S.; Bitar, F.F. Cardiac growth patterns in response to chronic hypoxia in a neonatal rat model mimicking cyanotic heart disease. Exp. Clin. Cardiol. 2003, 8, 189–194. [Google Scholar]
- Kantores, C.; McNamara, P.J.; Teixeira, L.; Engelberts, D.; Murthy, P.; Kavanagh, B.P.; Jankov, R.P. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L912–L922. [Google Scholar] [CrossRef]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Eckardt, K.U.; Bernhardt, W.M.; Weidemann, A.; Warnecke, C.; Rosenberger, C.; Wiesener, M.S.; Willam, C. Role of hypoxia in the pathogenesis of renal disease. Kidney Int. Suppl. 2005, 99, S46–S51. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Y.; Liu, F.; Tang, X.; Fu, H.; Mao, J. Renoprotective Role of Hypoxia-Inducible Factors and the Mechanism. Kidney Dis. 2022, 8, 44–56. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Gubhaju, L.; Moore, L.; Kent, A.L.; Dahlstrom, J.E.; Horne, R.S.; Hoy, W.E.; Bertram, J.F.; Black, M.J. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J. Am. Soc. Nephrol. 2011, 22, 1365–1374. [Google Scholar] [CrossRef] [Green Version]
- Rookmaaker, M.B.; Joles, J.A. The nephron number counts—From womb to tomb. Nephrol. Dial. Transplant. 2013, 28, 1325–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, W.M.; Schmitt, R.; Rosenberger, C.; Munchenhagen, P.M.; Grone, H.J.; Frei, U.; Warnecke, C.; Bachmann, S.; Wiesener, M.S.; Willam, C.; et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006, 69, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez, M.G.; Cabrera, I.; Serrano, D.J.; Sterin-Speziale, N. Cell proliferation and morphometric changes in the rat kidney during postnatal development. Anat. Embryol. 2002, 205, 431–440. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Pavlenko, T.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Silachev, D.N.; Sukhikh, G.T.; Zorov, D.B. The role of oxidative stress in acute renal injury of newborn rats exposed to hypoxia and endotoxin. FEBS J. 2017, 284, 3069–3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picut, C.A.; Dixon, D.; Simons, M.L.; Stump, D.G.; Parker, G.A.; Remick, A.K. Postnatal ovary development in the rat: Morphologic study and correlation of morphology to neuroendocrine parameters. Toxicol. Pathol. 2015, 43, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmody, J.B.; Charlton, J.R. Short-term gestation, long-term risk: Prematurity and chronic kidney disease. Pediatrics 2013, 131, 1168–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, D.R.; Rhee, C.J.; Elizondo, L.; Brady, K.M.; Rusin, C.G.; Acosta, S. Creatinine filtration kinetics in critically Ill neonates. Pediatr. Res. 2021, 89, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Colgan, S.P.; Shelley, C.S. Hypoxia: The Force that Drives Chronic Kidney Disease. Clin. Med. Res. 2016, 14, 15–39. [Google Scholar] [CrossRef] [Green Version]
- Zangaladze, A.; Cai, C.L.; Marcelino, M.; Aranda, J.V.; Beharry, K.D. Renal biomarkers of acute kidney injury in response to increasing intermittent hypoxia episodes in the neonatal rat. BMC Nephrol. 2021, 22, 299. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Q.; Wen, J.; Chen, T.; He, L.; Wang, Y.; Yin, J.; Wu, R.; Xue, R.; Li, S.; et al. Ischemic Duration and Frequency Determines AKI-to-CKD Progression Monitored by Dynamic Changes of Tubular Biomarkers in IRI Mice. Front. Physiol. 2019, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.L.; Liu, B.C. Hypoxia and Renal Tubulointerstitial Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 467–485. [Google Scholar]
- Adachi, S.; Zelenin, S.; Matsuo, Y.; Holtback, U. Cellular response to renal hypoxia is different in adolescent and infant rats. Pediatr. Res. 2004, 55, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubauer, J.A.; Sunderram, J. Heme oxygenase-1 and chronic hypoxia. Respir. Physiol. Neurobiol. 2012, 184, 178–185. [Google Scholar] [CrossRef]
- Shu, S.; Wang, Y.; Zheng, M.; Liu, Z.; Cai, J.; Tang, C.; Dong, Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, A.; Scholz, C.C.; de Seigneux, S. Hypoxia in chronic kidney disease: Towards a paradigm shift? Nephrol. Dial. Transplant. 2021, 36, 1782–1790. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Boor, P.; Ostendorf, T.; Floege, J. Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 2010, 6, 643–656. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Quaggin, S.E.; Vaughan, D.E. Molecular basis of organ fibrosis: Potential therapeutic approaches. Exp. Biol. Med. 2013, 238, 461–481. [Google Scholar] [CrossRef]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and chronic kidney disease. Contrib. Nephrol. 2013, 180, 47–63. [Google Scholar] [PubMed] [Green Version]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.R.; Holt, S.G.; Hewitson, T.D. alphaKlotho-FGF23 interactions and their role in kidney disease: A molecular insight. Cell Mol. Life Sci. 2019, 76, 4705–4724. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Tan, S.J.; Holt, S.G.; Hewitson, T.D. FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci. Rep. 2017, 7, 3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Ma, S.; Zheng, C.; Wang, Q.; Lin, H.; Chen, Z.; Xie, J.; Chen, L.; Chen, K.; Wang, Y.; et al. Excessive fibroblast growth factor 23 promotes renal fibrosis in mice with type 2 cardiorenal syndrome. Aging 2021, 13, 2982–3009. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Holt, S.G.; Hewitson, T.D. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int. J. Biochem. Cell Biol. 2017, 92, 63–78. [Google Scholar] [CrossRef]
- Uddin, M.J.; Kim, E.H.; Hannan, M.A.; Ha, H. Pharmacotherapy against Oxidative Stress in Chronic Kidney Disease: Promising Small Molecule Natural Products Targeting Nrf2-HO-1 Signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Sharma, A.K.; Muthuraju, S.; Banerjee, P.K.; Ilavazhagan, G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem. Int. 2006, 49, 709–716. [Google Scholar] [CrossRef]
- Ramanathan, L.; Gozal, D.; Siegel, J.M. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J. Neurochem. 2005, 93, 47–52. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Liu, G.S.; Dusting, G.J.; Chan, E.C. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol. 2014, 2, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiose, A.; Kuroda, J.; Tsuruya, K.; Hirai, M.; Hirakata, H.; Naito, S.; Hattori, M.; Sakaki, Y.; Sumimoto, H. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 2001, 276, 1417–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiszt, M.; Kopp, J.B.; Varnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Lee, H.Y.; Park, C.G.; Kang, J.; Yu, S.L.; Choi, D.R.; Han, S.Y.; Park, M.H.; Cho, S.; Lee, S.Y.; et al. Oxidative stress caused by activation of NADPH oxidase 4 promotes contrast-induced acute kidney injury. PLoS ONE 2018, 13, e0191034. [Google Scholar] [CrossRef] [Green Version]
- Gorin, Y. Nox4 as a potential therapeutic target for treatment of uremic toxicity associated to chronic kidney disease. Kidney Int. 2013, 83, 541–543. [Google Scholar] [CrossRef] [Green Version]
- Cuarental, L.; Sucunza-Sáenz, D.; Valiño-Rivas, L.; Fernandez-Fernandez, B.; Sanz, A.B.; Ortiz, A.; Vaquero, J.J.; Sanchez-Niño, M.D. MAP3K kinases and kidney injury. Nefrologia 2019, 39, 568–580. [Google Scholar] [CrossRef]
- Adhikary, L.; Chow, F.; Nikolic-Paterson, D.J.; Stambe, C.; Dowling, J.; Atkins, R.C.; Tesch, G.H. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia 2004, 47, 1210–1222. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, H.; Chen, F.; Wang, Y.; Ma, J.; Wang, G. The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp. Ther. Med. 2019, 17, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Moe, K.T.; Yin, N.O.; Naylynn, T.M.; Khairunnisa, K.; Wutyi, M.A.; Gu, Y.; Atan, M.S.; Wong, M.C.; Koh, T.H.; Wong, P. Nox2 and Nox4 mediate tumour necrosis factor-alpha-induced ventricular remodelling in mice. J. Cell Mol. Med. 2011, 15, 2601–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ignazio, L.; Rocha, S. Hypoxia Induced NF-kappaB. Cells 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, S.; Lin, L.; Hu, K. NF-kappaB and tPA Signaling in Kidney and Other Diseases. Cells 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrissey, J.; Klahr, S. Transcription factor NF-kappaB regulation of renal fibrosis during ureteral obstruction. Semin. Nephrol. 1998, 18, 603–611. [Google Scholar]
- Guijarro, C.; Egido, J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001, 59, 415–424. [Google Scholar] [CrossRef] [Green Version]





| Parameter/Group | Normoxia | Hypoxia |
|---|---|---|
| pH | 7.38 ± 0.02 | 7.35 ± 0.06 |
| Glucose (mg/dL) | 118.20 ± 21.81 | 124.67 ± 15.04 |
| Creatinine (mg/dL) | 0.51 ± 0.03 | 0.71 ± 0.09 * |
| Lactate (mmol/L) | 2.14 ± 0.69 | 4.12 ± 0.45 * |
| pCO2 (mmHg) | 49.18 ± 3.39 | 50.83 ± 10.19 |
| pO2 (mmHg) | 56.68 ± 4.09 | 27.77 ± 6.72 * |
| cTCO2 (mmol/L) | 30.82 ± 1.25 | 29.00 ± 1.74 |
| cSO2 (%) | 87.94 ± 2.53 | 46.93 ± 17.41 * |
| Sodium (mmol/L) | 128.00 ± 0.71 | 127.00 ± 1.73 |
| Potassium (mmol/L) | 6.84 ± 0.51 | 11.17 ± 1.44 * |
| Chloride (mmol/L) | 97.60 ± 2.19 | 102.00 ± 1.73 * |
| Calcium (mmol/L) | 1.49 ± 0.08 | 1.36 ± 0.07 |
| Hematocrit (%) | 22.00 ± 1.22 | 35.67 ± 0.58 * |
| cHgb (g/dL) | 7.50 ± 0.41 | 12.17 ± 0.21 * |
| cHCO3− (mmol/L) | 29.32 ± 1.16 | 27.43 ± 1.46 |
| BE(ecf) (mmol/L) | 4.26 ± 1.09 | 1.77 ± 0.55 * |
| BE(b) (mmol/L) | 3.76 ± 0.96 | 1.03 ± 0.32 * |
| AGapK (mmol/L) | 7.80 ± 1.64 | 8.60 ± 1.40 |
| AGap (mmol/L) | 1.00 ± 2.35 | −2.33 ± 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.-T.; Chen, B.-H.; Chen, H.-H.; Lee, J.-C.; Kuo, T.-J.; Chiu, H.-C.; Lu, W.-H. Hypoxia-Induced Kidney Injury in Newborn Rats. Toxics 2023, 11, 260. https://doi.org/10.3390/toxics11030260
Chu Y-T, Chen B-H, Chen H-H, Lee J-C, Kuo T-J, Chiu H-C, Lu W-H. Hypoxia-Induced Kidney Injury in Newborn Rats. Toxics. 2023; 11(3):260. https://doi.org/10.3390/toxics11030260
Chicago/Turabian StyleChu, Yi-Ting, Bo-Hau Chen, Hsin-Hung Chen, Jui-Chen Lee, Tzu-Jiun Kuo, Hsiang-Chin Chiu, and Wen-Hsien Lu. 2023. "Hypoxia-Induced Kidney Injury in Newborn Rats" Toxics 11, no. 3: 260. https://doi.org/10.3390/toxics11030260