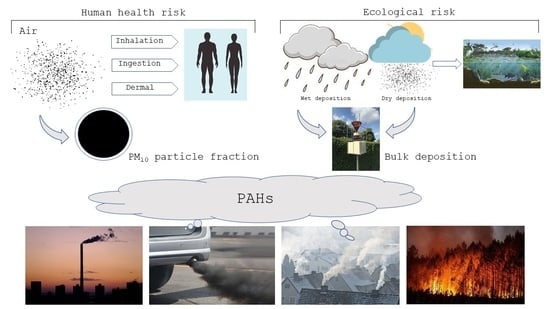
Carcinogenic Activity and Risk Assessment of PAHs in Ambient Air: PM10 Particle Fraction and Bulk Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analysis of PAHs in PM10 Particle Fraction
2.3. Analysis of PAHs in Bulk Deposition Samples
2.4. Statistical Analysis
2.5. Cancer Risk Assessment of PAHs
2.6. Ecological Risk Assessment of PAHs
3. Results and Discussion
3.1. Carcinogenic Activity of PAHs in Atmospheric Particulate Matter and Bulk Deposition
3.2. Risk Assessment of PAHs
3.2.1. Human Health Risk Assessment
3.2.2. Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lara, R.; Suárez-Peña, B.; Megido, L.; Negral, L.; Rodríguez-Iglesias, J.; Fernández-Nava, Y.; Castrillón, L. Health Risk Assessment of Potentially Toxic Elements in the Dry Deposition Fraction of Settleable Particulate Matter in Urban and Suburban Locations in the City of Gijón, Spain. J. Environ. Chem. Eng. 2021, 9, 106794. [Google Scholar] [CrossRef]
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent. Toxics 2017, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- IARC Agents Classified by the IARC Monographs, Volumes 1–132. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 28 December 2022).
- IARC. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 92, ISBN 978 92 832 1292 8. [Google Scholar]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic Polycyclic Aromatic Hydrocarbon-DNA Adducts and Mechanism of Action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to Polycyclic Aromatic Hydrocarbons with Special Focus on Cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Farmer, P.B.; Singh, R.; Kaur, B.; Sram, R.J.; Binkova, B.; Kalina, I.; Popov, T.A.; Garte, S.; Taioli, E.; Gabelova, A.; et al. Molecular Epidemiology Studies of Carcinogenic Environmental Pollutants: Effects of Polycyclic Aromatic Hydrocarbons (PAHs) in Environmental Pollution on Exogenous and Oxidative DNA Damage. Mutat. Res. Rev. Mutat. Res. 2003, 544, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.C. Linking DNA Adduct Formation and Human Cancer Risk in Chemical Carcinogenesis. Environ. Mol. Mutagen. 2016, 57, 499–507. [Google Scholar] [CrossRef]
- Boström, C.E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer Risk Assessment, Indicators, and Guidelines for Polycyclic Aromatic Hydrocarbons in the Ambient Air. Environ. Health Perspect. 2002, 110, 451–488. [Google Scholar] [CrossRef] [Green Version]
- Bach, P.B.; Kelley, M.J.; Tate, R.C.; McCrory, D.C. Screening for Lung Cancer: A Review of the Current Literature. Chest 2003, 123, 72S–82S. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A Review of Airborne Polycyclic Aromatic Hydrocarbons ( PAHs ) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer Risk from Occupational and Environmental Exposure to Polycyclic Aromatic Hydrocarbons. Cancer Causes Control 1997, 8, 444–472. [Google Scholar] [CrossRef]
- Latif, I.K.; Karim, A.J.; Zuki, A.B.Z.; Zamri-Saad, M.; Niu, J.P.; Noordin, M.M. Pulmonary Modulation of Benzo[a]Pyrene-Induced Hemato-and Hepatotoxicity in Broilers. Poult. Sci. 2010, 89, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Rebbeck, T.R. Bridging the Gap between Biologic, Individual, and Macroenvironmental Factors in Cancer: A Multilevel Approach. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Hecht, S.S. Tobacco Smoke Carcinogens and Lung Cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Zhang, L.; Cui, Y.; Cheng, M.; Guo, L.; Liu, M.; Chen, L. Particle Dry Deposition of Polycyclic Aromatic Hydrocarbons and Its Risk Assessment in a Typical Coal-Polluted and Basin City, Northern China. Atmos. Pollut. Res. 2017, 8, 1081–1089. [Google Scholar] [CrossRef]
- Šimić, I.; Mendaš, G.; Pehnec, G. An Optimized Sample Preparation and Analysis Method for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in the Atmospheric Bulk Deposition Samples. J. Chromatogr. A 2020, 1633, 461599. [Google Scholar] [CrossRef]
- Jakovljević, I.; Šimić, I.; Mendaš, G.; Sever Štrukil, Z.; Žužul, S.; Gluščić, V.; Godec, R.; Pehnec, G.; Bešlić, I.; Milinković, A.; et al. Pollution Levels and Deposition Processes of Airborne Organic Pollutants over the Central Adriatic Area: Temporal Variabilities and Source Identification. Mar. Pollut. Bull. 2021, 172, 112873. [Google Scholar] [CrossRef]
- Dämmgen, U.; Erisman, J.W.; Cape, J.N.; Grünhage, L.; Fowler, D. Practical Considerations for Addressing Uncertainties in Monitoring Bulk Deposition. Environ. Pollut. 2005, 134, 535–548. [Google Scholar] [CrossRef]
- Feng, D.; Liu, Y.; Gao, Y.; Zhou, J.; Zheng, L.; Qiao, G.; Ma, L.; Lin, Z.; Grathwohl, P. Atmospheric Bulk Deposition of Polycyclic Aromatic Hydrocarbons in Shanghai: Temporal and Spatial Variation, and Global Comparison. Environ. Pollut. 2017, 230, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, M.; Verta, M.; Salo, S.; Vuorenmaa, J.; Kiviranta, H. Atmospheric Bulk Deposition of Polychlorinated and Polychlorinated Biphenyls in Finland. J. Mar. Sci. Eng. 2016, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Esen, F.; Siddik Cindoruk, S.; Tasdemir, Y. Bulk Deposition of Polycyclic Aromatic Hydrocarbons (PAHs) in an Industrial Site of Turkey. Environ. Pollut. 2008, 152, 461–467. [Google Scholar] [CrossRef]
- Hussain, K.; Rahman, M.; Prakash, A.; Sarma, K.P.; Hoque, R.R. Atmospheric Bulk Deposition of PAHs over Brahmaputra Valley: Characteristics and Influence of Meteorology. Aerosol Air Qual. Res. 2016, 16, 1675–1689. [Google Scholar] [CrossRef] [Green Version]
- Galbán-Malagón, C.; Cabrerizo, A.; Caballero, G.; Dachs, J. Atmospheric Occurrence and Deposition of Hexachlorobenzene and Hexachlorocyclohexanes in the Southern Ocean and Antarctic Peninsula. Atmos. Environ. 2013, 80, 41–49. [Google Scholar] [CrossRef]
- Zhao, R.; Cui, K.; Wang, W.; Wang, L.-C.; Wang, Y.-F. Sensitivity Analyses for the Atmospheric Dry Deposition of Total PCDD/Fs-TEQ for Handan and Kaifeng Cities, China. Aerosol Air Qual. Res. 2018, 18, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Vassura, I.; Passarini, F.; Ferroni, L.; Bernardi, E.; Morselli, L. PCDD/Fs Atmospheric Deposition Fluxes and Soil Contamination Close to a Municipal Solid Waste Incinerator. Chemosphere 2011, 83, 1366–1373. [Google Scholar] [CrossRef]
- Arellano, L.; Fernández, P.; Van Drooge, B.L.; Rose, N.L.; Nickus, U.; Thies, H.; Stuchlík, E.; Camarero, L.; Catalan, J.; Grimalt, J.O.; et al. Drivers of Atmospheric Deposition of Polycyclic Aromatic Hydrocarbons at European High-Altitude Sites. Atmos. Chem. Phys. 2018, 18, 16081–16097. [Google Scholar] [CrossRef] [Green Version]
- Souza, D.; Heitmann, D.; Reifenha, W.; Meire, R.O.; Santos, L.S.; Torres, M.; Malm, O.; Ko, W. Persistent Organic Pollutants in Atmospheric Deposition and Biomonitoring with Tillandsia Usneoides (L.) in an Industrialized Area in Rio de Janeiro State, Southeast Brazil—Part II: PCB and PAH. Chemosphere 2007, 67, 1736–1745. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, L.; Xu, X.; Yu, Y.; Li, Y.; Zhang, H.; Xu, Y. Atmospheric Transport and Bulk Deposition of Organochlorine Compounds at Leigongshan Nature Reserve in Southwestern China. Aerosol Air Qual. Res. 2019, 19, 1483–1492. [Google Scholar] [CrossRef] [Green Version]
- Menichini, E.; Barbera, S.; Merli, F.; Settimo, G.; Viviano, G. Atmospheric Bulk Deposition of Carcinogenic PAHs in a Rural Area in Southern Italy. Polycycl. Aromat. Compd. 2006, 26, 253–263. [Google Scholar] [CrossRef]
- Hansson, K.; Cousins, A.P.; Brorström, E. Atmospheric Concentrations in Air and Deposition Fluxes of POPs at Råö and Pallas, Trends and Seasonal and Spatial Variations; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2006. [Google Scholar]
- Bari, M.A.; Kindzierski, W.B.; Cho, S. A Wintertime Investigation of Atmospheric Deposition of Metals and Polycyclic Aromatic Hydrocarbons in the Athabasca Oil Sands Region, Canada. Sci. Total Environ. 2014, 485–486, 180–192. [Google Scholar] [CrossRef]
- Godec, R.; Jakovljević, I.; Davila, S.; Šega, K.; Bešlić, I.; Rinkovec, J.; Pehnec, G. Air Pollution Levels near Crossroads with Different Traffic Density and the Estimation of Health Risk. Environ. Geochem. Health 2021, 43, 3935–3952. [Google Scholar] [CrossRef]
- Pehnec, G.; Jakovljević, I.; Godec, R.; Sever Štrukil, Z.; Žero, S.; Huremović, J.; Džepina, K. Carcinogenic Organic Content of Particulate Matter at Urban Locations with Different Pollution Sources. Sci. Total Environ. 2020, 734, 139414. [Google Scholar] [CrossRef]
- Jakovljević, I.; Pehnec, G.; Šišović, A.; Vađić, V.; Davila, S.; Godec, R. Concentrations of PAHs and Other Gaseous Pollutants in the Atmosphere of a Rural Area. J. Environ. Sci. Heal. Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 707–713. [Google Scholar] [CrossRef]
- Jakovljević, I.; Pehnec, G.; Vadjić, V.; Šišović, A.; Davila, S.; Bešlić, I. Carcinogenic Activity of Polycyclic Aromatic Hydrocarbons Bounded on Particle Fraction. Environ. Sci. Pollut. Res. 2015, 22, 15931–15940. [Google Scholar] [CrossRef]
- Šišović, A.; Pehnec, G.; Jakovljević, I.; Šilović Hujić, M.; Vacrossed D Signić, V.; Bešlić, I. Polycyclic Aromatic Hydrocarbons at Different Crossroads in Zagreb, Croatia. Bull. Environ. Contam. Toxicol. 2012, 88, 438–442. [Google Scholar] [CrossRef]
- Pehnec, G.; Jakovljević, I. Carcinogenic Potency of Airborne Polycyclic Aromatic Hydrocarbons in Relation to the Particle Fraction Size. Int. J. Environ. Res. Public Health 2018, 15, 2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakovljević, I.; Štrukil, Z.S.; Godec, R.; Bešlić, I.; Davila, S.; Lovrić, M.; Pehnec, G. Pollution Sources and Carcinogenic Risk of PAHs in PM1 Particle Fraction in an Urban Area. Int. J. Environ. Res. Public Health 2020, 17, 9587. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment. Arch. Environ. Contam. Toxicol. 2019, 76, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, J.; Ma, Q.; Chen, Y. Human Health and Ecological Risk Assessment of 16 Polycyclic Aromatic Hydrocarbons in Drinking Source Water from a Large Mixed-Use Reservoir. Int. J. Environ. Res. Public Health 2015, 12, 13956–13969. [Google Scholar] [CrossRef] [Green Version]
- Olayinka, O.O.; Adewusi, A.A.; Olarenwaju, O.O.; Aladesida, A.A. Concentration of Polycyclic Aromatic Hydrocarbons and Estimated Human Health Risk of Water Samples Around Atlas Cove, Lagos, Nigeria. J. Health Pollut. 2018, 8, 181210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, M.; Dai, Y.; Luo, Y.; Zhang, S. Health and Ecotoxicological Risk Assessment for Human and Aquatic Organism Exposure to Polycyclic Aromatic Hydrocarbons in the Baiyangdian Lake. Environ. Sci. Pollut. Res. 2021, 28, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Konan, B.R.; Yoboue, V.; Adiaffi, B.; Diaby, M.; Oga, Y.M.S.; Bakayoko, A.; Gnamien, S.; Keita, S.; Bahino, J.; Ossohou, M. Source of Polycyclic Aromatic Hydrocarbons (PAHs) in Rainwater and Effect on the Health of the Population: The Case of the District of Abidjan in the South of Ivory Coast. J. Water Health 2022, 20, 41. [Google Scholar] [CrossRef]
- EEA Air Quality in Europe. 2022. Available online: https://www.eea.europa.eu/publications/status-of-air-quality-in-Europe-2022/europes-air-quality-status-2022 (accessed on 29 December 2022).
- EEA. Air Quality in Europe—2020 Report; European Environment Agency: Copenhagen, Denmark, 2020; ISBN 978-92-9480-292-7.
- Pehnec, G.; Jakovljević, I.; Šišović, A.; Bešlić, I.; Vadić, V. Influence of Ozone and Meteorological Parameters on Levels of Polycyclic Aromatic Hydrocarbons in the Air. Atmos. Environ. 2016, 131, 263–268. [Google Scholar] [CrossRef]
- US EPA. Risk Assessment Guidance for Superfund (RAGS). Volume I. Human Health Evaluation Manual (HHEM). Part E. Supplemental Guidance for Dermal Risk Assessment; U.S. EPA: Washington, DC, USA, 2004; Volume 1, ISBN EPA/540/1-89/002.
- U.S. EPA. Human Health Evaluation Manual, Supplemental Guidance: Update of Standard Default Exposure Factors; 2014; Volume OSWER Dire. [Google Scholar]
- Sobhanardakani, S. Human Health Risk Assessment of Potentially Toxic Heavy Metals in the Atmospheric Dust of City of Hamedan, West of Iran. Environ. Sci. Pollut. Res. 2018, 25, 28086–28093. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Health Risk Assessment of Heavy Metal Exposure to Street Dust in the Zinc Smelting District, Northeast of China. Sci. Total Environ. 2010, 408, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.S.; Ding, J.; Xu, B.; Wang, Y.J.; Li, H.B.; Yu, S. Incorporating Bioaccessibility into Human Health Risk Assessments of Heavy Metals in Urban Park Soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, X.; Han, X.; Zhao, N. Ecological and Health Risk Assessment of Metal in Resuspended Particles of Urban Street Dust from an Industrial City in China. Curr. Sci. 2015, 108, 72–79. [Google Scholar]
- Li, H.; Qian, X.; Hu, W.; Wang, Y.; Gao, H. Chemical Speciation and Human Health Risk of Trace Metals in Urban Street Dusts from a Metropolitan City, Nanjing, SE China. Sci. Total Environ. 2013, 456–457, 212–221. [Google Scholar] [CrossRef]
- Franco, C.F.J.; de Resende, M.F.; de Almeida Furtado, L.; Brasil, T.F.; Eberlin, M.N.; Netto, A.D.P. Polycyclic Aromatic Hydrocarbons (PAHs) in Street Dust of Rio de Janeiro and Niterói, Brazil: Particle Size Distribution, Sources and Cancer Risk Assessment. Sci. Total Environ. 2017, 599–600, 305–313. [Google Scholar] [CrossRef]
- U.S. EPA. Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants; U.S. EPA: Washington, DC, USA, 2005.
- Chu, M.M.L.; Chen, C.W. The Evaluation and Estimation of Potential Carcinogenic Risks of Polynuclear Aromatic Hydrocarbons (PAH); U.S. EPA: Washington, DC, USA, 1985.
- Nisbet, I.C.T.; LaGoy, P.K. Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Collins, J.F.; Brown, J.P.; Alexeeff, G.V.; Salmon, A.G. Potency Equivalency Factors for Some Polycyclic Aromatic Hydrocarbons and Polycyclic Aromatic Hydrocarbon Derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef]
- U.S. EPA. Risk Assessment Guidance for Superfund (RAGS) Volume III—Part A: Process for Conducting Probabilistic Risk Assessment; U.S. EPA: Washington, DC, USA, 2001; Volume III, pp. 1–385.
- Sánchez-Piñero, J.; Moreda-Piñeiro, J.; Turnes-Carou, I.; Fernández-Amado, M.; Muniategui-Lorenzo, S.; López-Mahía, P. Polycyclic Aromatic Hydrocarbons in Atmospheric Particulate Matter (PM10) at a Southwestern Europe Coastal City: Status, Sources and Health Risk Assessment. Air Qual. Atmos. Heal. 2021, 14, 1325–1339. [Google Scholar] [CrossRef]
- U.S. EPA. Risk Assessment Guidance for Superfund. Volume I Human Health Evaluation Manual (Part A); U.S. EPA: Washington, DC, USA, 1989; Volume I, p. 289.
- Kalf, D.F.; Crommentuijn, T.; Van de Plassche, E.J. Environmental Quality Objectives for 10 Polycyclic Aromatic Hydrocarbons (PAHS). Ecotoxicol. Environ. Saf. 1997, 36, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, J.; Luan, Y.; Li, Y.; Ma, M.; Xu, J.; Han, S. Distribution and Ecosystem Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Luan River, China. Ecotoxicology 2010, 19, 827–837. [Google Scholar] [CrossRef]
- Norramit, P.; Cheevaporn, V.; Itoh, N.; Tanaka, K. Characterizatlon and Carcinogenic Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Respirable Fraction of Airborne Particles in the Bangkok Metropolitan Area. J. Health Sci. 2005, 51, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Menichini, E.; Monfredini, F.; Merli, F. The Temporal Variability of the Profile of Carcinogenic Polycyclic Aromatic Hydrocarbons in Urban Air: A Study in a Medium Traffic Area in Rome, 1993-1998. Atmos. Environ. 1999, 33, 3739–3750. [Google Scholar] [CrossRef]
- Wada, M.; Kido, H.; Kishikawa, N.; Tou, T.; Tanaka, M.; Tsubokura, J.; Shironita, M.; Matsui, M.; Kuroda, N.; Nakashima, K. Assessment of Air Pollution in Nagasaki City: Determination of Polycyclic Aromatic Hydrocarbons and Their Nitrated Derivatives, and Some Metals. Environ. Pollut. 2001, 115, 139–147. [Google Scholar] [CrossRef]
- Chen, P.; Kang, S.; Li, C.; Li, Q.; Yan, F.; Gou, J.; Ji, Z.; Zhang, Q.; Hu, Z.; Tripathee, L.; et al. Source Apportionment and Risk Assessment of Atmospheric Polycyclic Aromatic Hydrocarbons in Lhasa, Tibet, China. Aerosol Air Qual. Res. 2018, 18, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Nadali, A.; Leili, M.; Bahrami, A.; Karami, M.; Afkhami, A. Phase Distribution and Risk Assessment of PAHs in Ambient Air of Hamadan, Iran. Ecotoxicol. Environ. Saf. 2021, 209, 111807. [Google Scholar] [CrossRef]
- Xing, W.; Zhang, L.; Yang, L.; Zhou, Q.; Zhang, X.; Toriba, A.; Hayakawa, K.; Tang, N. Characteristics of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and Nitro-Polycyclic Aromatic Hydrocarbons at a Roadside Air Pollution Monitoring Station in Kanazawa, Japan. Int. J. Environ. Res. Public Health 2020, 17, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garban, B.; Blanchoud, H.; Motelay-Massei, A.; Chevreuil, M.; Ollivon, D. Atmospheric Bulk Deposition of PAHs onto France: Trends from Urban to Remote Sites. Atmos. Environ. 2002, 36, 5395–5403. [Google Scholar] [CrossRef]
- Moon, H.B.; Kannan, K.; Lee, S.J.; Ok, G. Atmospheric Deposition of Polycyclic Aromatic Hydrocarbons in an Urban and a Suburban Area of Korea from 2002 to 2004. Arch. Environ. Contam. Toxicol. 2006, 51, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Carcinogenic Potential, Levels and Sources of Polycyclic Aromatic Hydrocarbon Mixtures in Indoor and Outdoor Environments and Their Implications for Air Quality Standards. Environ. Int. 2011, 37, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, C.; Lyons, J.M.; Friedlander, S.K. Size Distributions of Polycyclic Aromatic Hydrocarbons and Elemental Carbon. 1. Sampling, Measurement Methods, and Source Characterization. Environ. Sci. Technol. 1994, 28, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, M.; Çabuk, H. Particle-Associated Polycyclic Aromatic Hydrocarbons in the Atmospheric Environment of Zonguldak, Turkey. Sci. Total Environ. 2008, 405, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Andreou, G.; Rapsomanikis, S. Polycyclic Aromatic Hydrocarbons and Their Oxygenated Derivatives in the Urban Atmosphere of Athens. J. Hazard. Mater. 2009, 172, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and Respirable Particle Definitions for.Pdf. Part. Fibre Toxicol. 2013, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siudek, P. Polycyclic Aromatic Hydrocarbons in Coarse Particles (PM10) over the Coastal Urban Region in Poland: Distribution, Source Analysis and Human Health Risk Implications. Chemosphere 2023, 311, 137130. [Google Scholar] [CrossRef] [PubMed]
- Majewski, G.; Widziewicz, K.; Rogula-Kozłowska, W.; Rogula-Kopiec, P.; Kociszewska, K.; Rozbicki, T.; Majder-Łopatka, M.; Niemczyk, M. PM Origin or Exposure Duration? Health Hazards from PM-Bound Mercury and PM-Bound PAHs among Students and Lecturers. Int. J. Environ. Res. Public Health 2018, 15, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Simonich, S.; Giri, B.; Chang, Y.; Zhang, Y.; Jia, Y.; Tao, S.; Wang, R.; Wang, B.; Li, W.; et al. Atmospheric Concentrations and Air-Soil Gas Exchange of Polycyclic Aromatic Hydrocarbons (PAHs) in Remote, Rural Village and Urban Areas of Beijing-Tianjin Region, North China. Sci. Total Environ. 2011, 409, 2942–2950. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Chen, W.; Liao, X.; Wang, M.; Ouyang, Z.; Jiao, W.; Bai, Y. Polycyclic Aromatic Hydrocarbons in Urban Soils of Beijing: Status, Sources, Distribution and Potential Risk. Environ. Pollut. 2011, 159, 802–808. [Google Scholar] [CrossRef]
- Wright, L.P.; Zhang, L.; Cheng, I.; Aherne, J.; Wentworth, G.R. Impacts and Effects Indicators of Atmospheric Deposition of Major Pollutants to Various Ecosystems—A Review. Aerosol Air Qual. Res. 2018, 18, 1953–1992. [Google Scholar] [CrossRef] [Green Version]



| Variable Definition | Symbol | Units | Children | Adults |
|---|---|---|---|---|
| Body weight | BW | kg | 20 | 80 |
| Exposure frequency | EF | day year−1 | 350 | 350 |
| Exposure duration | ED | year | 6 | 40 |
| Ingestion rate | IRs | mg day−1 | 200 | 100 |
| Dermal exposure area | SA | cm2 | 3107 | 4615 |
| Dermal adherence factor | AF | mg cm−2 | 0.20 | 0.07 |
| Dermal absorption factor | ABS | unitless | 0.13 | 0.13 |
| Averaging life span | AT | day | 25550 | 25550 |
| Inhalation rate | IRα | m3 h−1 | 1.3 | 3.3 |
| Exposure time | ET | h day−1 | 8 | 8 |
| Particle emission factor | PEF | m3 kg−1 | 1.36 × 109 | 1.36 × 109 |
| Ingestion carcinogenic slope factor | CSFing | (mg kg−1 day−1)−1 | 7.3 | 7.3 |
| Dermal carcinogenic slope factor | CSFderm | (mg kg−1 day−1)−1 | 25 | 25 |
| Inhalation carcinogenic slope factor | CSFinh | (mg kg−1 day−1)−1 | 3.14 | 3.14 |
| Children | Adults | ||||
|---|---|---|---|---|---|
| Central | Worst | Central | Worst | ||
| Cold period | Ding | 67.110 | 196.592 | 55.925 | 163.827 |
| Dder | 27.107 | 79.405 | 23.487 | 68.801 | |
| Dinh | 0.003 | 0.008 | 0.011 | 0.032 | |
| Warm period | Ding | 6.798 | 86.528 | 5.665 | 72.106 |
| Dder | 2.746 | 34.949 | 2.379 | 30.282 | |
| Dinh | 0.0003 | 0.003 | 0.001 | 0.014 | |
| Children | Adults | ||||
|---|---|---|---|---|---|
| Central | Worst | Central | Worst | ||
| Cold period | Risking | 4.90 × 10−4 | 1.44 × 10−3 | 4.08 × 10−4 | 1.20 × 10−3 |
| Riskder | 6.78 × 10−4 | 1.99 × 10−3 | 5.87 × 10−4 | 1.72 × 10−3 | |
| Riskinh | 8.06 × 10−9 | 2.36 × 10−8 | 3.41 × 10−8 | 9.99 × 10−8 | |
| Risktot | 1.17 × 10−3 | 3.42 × 10−3 | 9.95 × 10−4 | 2.92 × 10−3 | |
| Warm period | Risking | 4.96 × 10−5 | 6.32 × 10−4 | 4.14 × 10−5 | 5.26 × 10−4 |
| Riskder | 6.86 × 10−5 | 8.74 × 10−4 | 5.95 × 10−5 | 7.57 × 10−4 | |
| Riskinh | 8.16 × 10−4 | 1.04 × 10−8 | 3.45 × 10−9 | 4.40 × 10−8 | |
| Risktot | 1.18 × 10−4 | 1.51 × 10−3 | 1.01 × 10−4 | 1.28 × 10−3 | |
| Individual PAHs | ∑PAHs | ||||
|---|---|---|---|---|---|
| RQNCs | RQMPCs | RQ∑PAHs(NCs) | RQ∑PAHs(MPCs) | ||
| Risk-free | 0 | Risk-free | 0 | ||
| Low-risk | ≥1; <800 | 0 | |||
| Moderate-risk | ≥1 | <1 | Moderate-risk1 | ≥800 | 0 |
| Moderate-risk2 | <800 | ≥1 | |||
| High-risk | ≥1 | High-risk | ≥800 | ≥1 | |
| PAHs | RQ(NCs) | RQ(MPCs) |
|---|---|---|
| Flu | 0.0 | 0.0 |
| Pyr | 0.1 | 0.0 |
| BaA | 13.5 | 0.1 |
| Chry | 0.1 | 0.0 |
| BbF | 21.8 | 0.2 |
| BkF | 2.2 | 0.0 |
| BaP | 14.4 | 0.1 |
| DahA | 0.3 | 0.0 |
| BghiP | 0.4 | 0.0 |
| IP | 3.4 | 0.0 |
| ∑PAHs | 55.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, I.; Smoljo, I.; Sever Štrukil, Z.; Pehnec, G. Carcinogenic Activity and Risk Assessment of PAHs in Ambient Air: PM10 Particle Fraction and Bulk Deposition. Toxics 2023, 11, 228. https://doi.org/10.3390/toxics11030228
Jakovljević I, Smoljo I, Sever Štrukil Z, Pehnec G. Carcinogenic Activity and Risk Assessment of PAHs in Ambient Air: PM10 Particle Fraction and Bulk Deposition. Toxics. 2023; 11(3):228. https://doi.org/10.3390/toxics11030228
Chicago/Turabian StyleJakovljević, Ivana, Iva Smoljo, Zdravka Sever Štrukil, and Gordana Pehnec. 2023. "Carcinogenic Activity and Risk Assessment of PAHs in Ambient Air: PM10 Particle Fraction and Bulk Deposition" Toxics 11, no. 3: 228. https://doi.org/10.3390/toxics11030228