GC-MS and LC-MS Pesticide Analysis of Black Teas Originating from Sri Lanka, Iran, Turkey, and India
Abstract
:1. Introduction
2. Materials and Methods
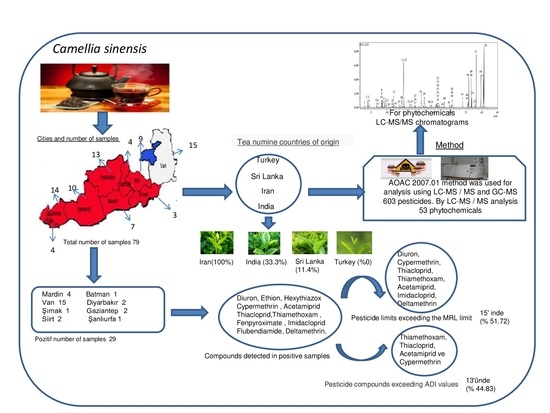
2.1. Collection and Preparation of Tea Samples for Analysis
2.2. Extraction of Tea Samples
2.3. LC-MS Determination of Pesticide Residues
2.4. GC-MS Determination of Pesticide Residues
3. Results
| City Where the Tea Sample was Taken | Origin of Tea Sample | Detected Pesticide Compounds | Results/Measurement | Measurement Limit (LOQ) | Uncertainty | Compliance with Regulation | Analysis Method |
|---|---|---|---|---|---|---|---|
| Mardin | Sri Lanka | Diuron | 0.016 ± 0.008 | 0.010 | 0.008 | Ok | LC-MS/MS |
| Mardin | Sri Lanka | Diuron | 0.021 ± 0.007 | 0.010 | 0.014 | Not ok | LC-MS/MS |
| Mardin | Sri Lanka | Diuron | 0.011 ± 0.006 | 0.010 | 0.005 | Ok | LC-MS/MS |
| Mardin | Sri Lanka | Diuron | 0.017 ± 0.006 | 0.010 | 0.011 | Not ok | LC-MS/MS |
| Van | Iran | Ethion | 0.013 ± 0.007 | 0.010 | 0.006 | Ok | GC-MS & GC MS/MS |
| Van | Iran | Cypermethrin | 0.014 ± 0.007 | 0.010 | 0.007 | Ok | GC-MS & GC MS/MS |
| Van | Iran | Diuron | 0.011 ± 0.006 | 0.010 | 0.005 | Ok | LC-MS/MS |
| Van | Iran | Ethion | 0.017 ± 0.009 | 0.010 | 0.008 | Ok | GC-MS & GC MS/MS |
| Van | Iran | Thiacloprid | 0.021 ± 0.011 | 0.010 | 0.010 | Ok | LC-MS/MS |
| Van | Iran | Thiamethoxam | 0.050 ± 0.025 | 0.010 | 0.025 | Not ok | LC-MS/MS |
| Van | Iran | Ethion | 0.019 ± 0.009 | 0.010 | 0.010 | Ok | GC-MS & GC MS/MS |
| Thiacloprid | 0.025 ± 0.013 | 0.010 | 0.012 | Not ok | LC-MS/MS | ||
| Thiamethoxam | 0.054 ± 0.021 | 0.010 | 0.033 | Not ok | LC-MS/MS | ||
| Van | Iran | Thiamethoxam | 0.069 ± 0.035 | 0.010 | 0.034 | Not ok | LC-MS/MS |
| Van | Iran | Cypermethrin | 0.014 ± 0.007 | 0.010 | 0.007 | Ok | GC-MS & GC MS/MS |
| Van | Iran | Ethion | 0.017 ± 0.009 | 0.010 | 0.008 | Ok | GC-MS & GC MS/MS |
| Van | Iran | Fenpyroximate | 0.012 ± 0.006 | 0.010 | 0.006 | Ok | LC-MS/MS |
| Van | Iran | Thiacloprid | 0.036 ± 0.018 | 0.010 | 0.018 | Not ok | LC-MS/MS |
| Van | Iran | Acetamiprid | 0.062 ± 0.031 | 0.010 | 0.031 | Not ok | LC-MS/MS |
| Cypermethrin | 0.027 ± 0.014 | 0.010 | 0.013 | Not ok | GC-MS & GC MS/MS | ||
| Van | Iran | Acetamiprid | 0.033 ± 0.017 | 0.010 | 0.016 | Not ok | LC-MS/MS |
| Cypermethrin | 0.017 ± 0.009 | 0.010 | 0.008 | Ok | GC-MS & GC MS/MS | ||
| Ethion | 0.012 ± 0.006 | 0.010 | 0.006 | Ok | GC-MS & GC MS/MS | ||
| Flubendiamide | 0.015 ± 0.008 | 0.010 | 0.007 | Ok | LC-MS/MS | ||
| Thiacloprid | 0.080 ± 0.040 | 0.010 | 0.040 | Not ok | LC-MS/MS | ||
| Thiamethoxam | 0.076 ± 0.038 | 0.010 | 0.038 | Not ok | LC-MS/MS | ||
| Van | Iran | Ethion | 0.019 ± 0.010 | 0.010 | 0.009 | Ok | GC-MS & GC MS/MS |
| Imidacloprid | 0.023 ± 0.012 | 0.010 | 0.011 | Not ok | LC-MS/MS | ||
| Thiacloprid | 0.058 ± 0.029 | 0.010 | 0.029 | Not ok | LC-MS/MS | ||
| Thiamethoxam | 0.076 ± 0.038 | 0.010 | 0.038 | Not ok | LC-MS/MS | ||
| Sirnak | Sri Lanka (Kuwait) | Diuron | 0.012 ± 0.006 | 0.010 | 0.006 | Ok | LC-MS/MS |
| Siirt | Sri Lanka | Ethion | 0.011 ± 0.006 | 0.010 | 0.005 | Ok | GC-MS & GC MS/MS |
| Thiacloprid | 0.011 ± 0.006 | 0.010 | 0.005 | Ok | LC-MS/MS | ||
| Thiamethoxam | 0.018 ± 0.009 | 0.010 | 0.009 | Ok | LC-MS/MS | ||
| Siirt | Iran | Diuron | 0.028 ± 0.014 | 0.010 | 0.014 | Not ok | LC-MS/MS |
| Diyarbakır | Sri Lanka | Deltamethrin | 0.029 ± 0.015 | 0.010 | 0.014 | Not ok | LC-MS/MS |
| Thiacloprid | 0.047 ± 0.024 | 0.010 | 0.023 | Not ok | LC-MS/MS | ||
| Thiamethoxam | 0.050 ± 0.025 | 0.010 | 0.025 | Not ok | LC-MS/MS | ||
| Diyarbakır | Iran | Hexythiazox | 0.015 ± 0.008 | 0.010 | 0.007 | Ok | LC-MS/MS |
| Thiacloprid | 0.027 ± 0.014 | 0.010 | 0.013 | Not ok | LC-MS/MS | ||
| Thiamethoxam | 0.052 ± 0.026 | 0.010 | 0.026 | Not ok | LC-MS/MS | ||
| Batman | Iran | Ethion | 0.032 ± 0.016 | 0.010 | 0.016 | Not ok | GC-MS & GC MS/MS |
| Thiacloprid | 0.086 ± 0.043 | 0.010 | 0.043 | Not ok | LC-MS/MS | ||
| Thiamethoxam | 0.076 ± 0.038 | 0.010 | 0.038 | Not ok | LC-MS/MS | ||
| Gaziantep | India | Ethion | 0.021 ± 0.011 | 0.010 | 0.010 | Ok | GC-MS & GC MS/MS |
| Thiacloprid | 0.012 ± 0.006 | 0.010 | 0.006 | Ok | LC-MS/MS | ||
| Thiamethoxam | 0.034 ± 0.017 | 0.010 | 0.017 | Not ok | LC-MS/MS | ||
| Gaziantep | Sri Lanka | Ethion | 0.020 ± 0.010 | 0.010 | 0.010 | Ok | GC-MS & GC MS/MS |
| Sanliurfa | Sri Lanka | Diuron | 0.011 ± 0.006 | 0.010 | 0.005 | Ok | LC-MS/MS |
| City Where the Tea Sample is Taken | Origin of Tea Sample | Detected Pesticide Compounds | Result-Measurement Uncertainty mg/kg | STMR × Dry Tea Consumed Daily (10 mg) | Brew Factor | * TMDI = STMR × Dry Tea Consumed Daily (10 mg) × Brew Factor | ADI (CODEX) |
|---|---|---|---|---|---|---|---|
| Mardin | Sri Lanka | Diuron | 0.008 | 0.08 | - | - | 0.01 |
| Mardin | Sri Lanka | Diuron | 0.014 | 0.14 | - | - | 0.01 |
| Mardin | Sri Lanka | Diuron | 0.005 | 0.05 | - | - | 0.01 |
| Mardin | Sri Lanka | Diuron | 0.011 | 0.11 | - | - | 0.01 |
| Van | Iran | Ethion | 0.006 | 0.06 | 0.025 | 0.015 | 0.02 |
| Van | Iran | Cypermethrin | 0.007 | 0.07 | 0.021 | 0.015 | 0.02 |
| Van | Iran | Diuron | 0.005 | 0.05 | - | - | 0.01 |
| Van | Iran | Ethion | 0.008 | 0.08 | 0.025 | 0.002 | 0.02 |
| Van | Iran | Thiacloprid | 0.010 | 0.10 | - | 0.01 | |
| Van | Iran | Thiamethoxam | 0.025 | 0.25 | 0.816 | 0.566 | 0.08 |
| Van | Iran | Ethion | 0.010 | 0.10 | 0.025 | 0.0025 | 0.02 |
| Thiacloprid | 0.012 | 0.12 | 0.497 | 0.06 | 0.01 | ||
| Thiamethoxam | 0.033 | 0.33 | 0.816 | 0.27 | 0.08 | ||
| Van | Iran | Thiamethoxam | 0.034 | 0.34 | 0.816 | 0.28 | 0.08 |
| Van | Iran | Cypermethrin | 0.007 | 0.07 | 0.021 | 0.015 | 0.02 |
| Van | Iran | Ethion | 0.008 | 0.08 | 0.025 | 0.0015 | 0.02 |
| Van | Iran | Fenpyroximate | 0.006 | 0.06 | - | - | 0.01 |
| Van | Iran | Thiacloprid | 0.018 | 0.18 | 0.497 | 0.09 | 0.01 |
| Van | Iran | Acetamiprid | 0.031 | 0.31 | 0.806 | 0.25 | 0.07 |
| Cypermethrin | 0.013 | 0.13 | 0.021 | 0.28 | 0.02 | ||
Van | Iran | Acetamiprid | 0.016 | 0.16 | 0.806 | 0.13 | 0.07 |
| Cypermethrin | 0.008 | 0.08 | 0.021 | 0.0017 | 0.02 | ||
| Ethion | 0.006 | 0.06 | 0.025 | 0.0015 | 0.02 | ||
| Flubendiamide | 0.007 | 0.07 | - | - | 0.02 | ||
| Thiacloprid | 0.040 | 0.4 | 0.497 | 0.2 | 0.01 | ||
| Thiamethoxam | 0.038 | 0.38 | 0.816 | 0.31 | 0.08 | ||
| Van | Iran | Ethion | 0.009 | 0.09 | 0.025 | 0.0023 | 0.02 |
| Imidacloprid | 0.011 | 0.11 | 0.42 | 0.05 | 0.06 | ||
| Thiacloprid | 0.029 | 0.29 | 0.497 | 0.14 | 0.01 | ||
| Thiamethoxam | 0.038 | 0.38 | 0.816 | 0.31 | 0.08 | ||
| Sirnak | Sri Lanka (Kuwait) ** | Diuron | 0.006 | 0.06 | - | - | 0.01 |
| Siirt | Sri Lanka | Ethion | 0.005 | 0.05 | 0.025 | 0.0013 | 0.02 |
| Thiacloprid | 0.005 | 0.05 | 0.497 | 0.025 | 0.01 | ||
| Thiamethoxam | 0.009 | 0.09 | 0.816 | 0.075 | 0.08 | ||
| Siirt | Iran | Diuron | 0.014 | 0.14 | - | - | 0.01 |
| Diyarbakır | Sri Lanka | Deltamethrin | 0.014 | 0.14 | 0.0046 | 0.0006 | 0.01 |
| Thiacloprid | 0.023 | 0.23 | 0.497 | 0.11 | 0.01 | ||
| Thiamethoxam | 0.025 | 0.25 | 0.816 | 0.204 | 0.08 | ||
| Diyarbakır | Iran | Hexythiazox | 0.007 | 0.7 | - | - | 0.03 |
| Thiacloprid | 0.013 | 0.13 | 0.497 | 0.06 | 0.01 | ||
| Thiamethoxam | 0.026 | 0.26 | 0.816 | 0.2 | 0.08 | ||
| Batman | Iran | Ethion | 0.016 | 0.16 | 0.025 | 0.004 | 0.02 |
| Thiacloprid | 0.043 | 0.43 | 0.497 | 0.2 | 0.01 | ||
| Thiamethoxam | 0.038 | 0.38 | 0.816 | 0.3 | 0.08 | ||
| Gaziantep | India | Ethion | 0.010 | 0.10 | 0.025 | 0.0025 | 0.02 |
| Thiacloprid | 0.006 | 0.06 | 0.497 | 0.03 | 0.01 | ||
| Thiamethoxam | 0.017 | 0.17 | 0.816 | 0.13 | 0.08 | ||
| Gaziantep | Sri Lanka | Ethion | 0.010 | 0.10 | 0.025 | 0.0025 | 0.02 |
| Sanliurfa | Sri Lanka | Diuron | 0.005 | 0.05 | - | - | 0.01 |
| Pesticides | CODEX MRL (mg/kg) | EU MRL (mg/kg) | USA * mg/kg | Canada * mg/kg | Australia * (mg/kg) | Japan * mg/kg | CODEX ADI mg/kg | Transfer Rate (%) | Water Solubility (mg/L) | Brew Factor ** |
|---|---|---|---|---|---|---|---|---|---|---|
| Diuron | - | 0.05 | - | - | - | - | 0.01 | 1.4–2.1 | 0.01 | 0.014–0.021 |
| Imidacloprid | - | 0.05 | - | - | - | - | 0.06 | 62.2–63.1 | 610 | 0.62–0.63 |
| Fenpyroximate | - | 8 | - | - | - | - | 0.01 | - | - | - |
| Acetamiprid | - | 0.05 * | 50 | - | - | 30 | 0.07 | 78.3–80.6 | 4200 | 0.78–0.81 |
| Cypermethrin | 20 (*15) | 0.5 | - | 0.5 | 20 | 0.02 | - | - | - | |
| Deltamethrin | 5 | 5 | - | 7 | 5 | 10 | 0.01 | 0.14–0.46 | 0.002 | 0.0014–0.0046 |
| Ethion | - | 3 | - | - | 5 | 0.3 | 0.02 | 2.25–2.5 | 2 | 0.022-–0.025 |
| Flubendiamide | 50 | 0.02 * | - | 0.02 | 0.02 | 40 | 0.02 | - | - | - |
| Hexythiazox | 15 | 4 | - | - | 4 | 35 | 0.03 | - | - | - |
| Thiamethoxam | 20 | 20 | 20 | - | 20 | 20 | 0.08 | 80.5–81.6 | 4100 | 0.81–0.82 |
| Thiacloprid | - | 10 | - | - | 10 | 30 | 0.01 | 49.7–50.0 | 184 | 0.49–0.50 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKay, D.L.; Blumberg, J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Elhadad, M.A.; Karavasiloglou, N.; Wulaningsih, W.; Tsilidis, K.K.; Tzoulaki, I.; Patel, C.J.; Rohrmann, S. Metabolites, nutrients, and lifestyle factors in relation to coffee consumption: An Environment-Wide Association Study. Nutrients 2020, 12, 19–1470. [Google Scholar] [CrossRef] [PubMed]
- Üstün, Ç.; Demirci, N. Historical development, and medical evaluation of the tea plant (Camellia sinensis L.). Mersin Univ. Fac. Med. Lokman Hekim Hist. Med. Folk. Med. J. 2013, 3, 5–12. [Google Scholar]
- Kurt, G.; Hacioğlu, H.K. Investigation of Tea Production of the World Countries and Turkey with Statistics. In Eastern Black Sea Industry Trade and Logistics Center II. Rize Development Symposium Tea Logistics Tourism Proceedings Book; Recep Tayyip Erdoğan University Publications, Rize, Turkey, 2013; pp. 39–64.
- Takim, K.; Aydemir, M.E. Aflatoxin analysis by LC-MS of local and imported black tea sold in Turkey. Int. J. Agric. Environ. Food Sci. 2021, 5, 640–644. [Google Scholar] [CrossRef]
- Amirahmadi, M.; Shoeibi, S.; Abdollahi, M.; Rastegar, H.; Khosrokhavar, R.; Hamedani, M.P. Monitoring of some pesticides residue in consumed tea in Tehran market. J. Environ. Health Sci. Eng. 2013, 10, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Gurusubramanian, G.; Rahman, A.; Sarmah, M.; Ray, S.; Bora, S. Pesticide usage pattern in tea ecosystem, their retrospects and alternative measures. J. Environ. Biol. 2008, 29, 813–826. [Google Scholar]
- Takım, K.; Aydemir, M.E. Pesticide Analysis by LC-MS and GC-MS in Leaky Tea Consumed in Sanliurfa. KSU J. Agric. Nat. 2018, 21, 650–664. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–553. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Pan, M.; Liu, X.; Lu, C. Evaluation of transfer rates of multiple pesticides from green tea into infusion using water as pressurized liquid extraction solvent and ultra-performance liquid chromatography tandem mass spectrometry. Food Chem. 2017, 216, 1–9. [Google Scholar] [CrossRef]
- Soyöz, M.; Özcelik, N. Cytogenetic effects of pesticides used in agricultural control. J. SDU Fac. Med. 2009, 10, 21–34. [Google Scholar] [CrossRef]
- Anonymous. Turkish Food Codex Regulation on Maximum Residue Limits of Pesticides T.C. Official Gazette (29899). Available online: https://www.resmigazete.gov.tr/eskiler/2016/11/20161125M1-1.htm (accessed on 28 March 2022).
- CAC. Joint FAO/WHO food standards program—Codex Alimentarius Commission. In Proceedings of the Report of the 48th Session of the Codex Committee on Pesticide Residues. 2016. Available online: https://www.fao.org/fao-who-codexalimentarius (accessed on 25 September 2022).
- Areo, O.M.; Olowoyo, J.O.; Sethoga, L.S.; Adebo, O.A.; Njobeh, P.B. Determination of pesticide residues in rooibos (Aspalathus linearis) teas in South Africa. Toxicol. Rep. 2022, 9, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Areo, O.M.; Abafe, O.A.; Gbashi, S.; Njobeh, P.B. Detection of multi-mycotoxins in rooibos and other consumed teas in South Africa by a modified QuEChERS method and ultra-high performance liquid chromatography tandem mass spectrometry. Food Control 2023, 143, 109255. [Google Scholar] [CrossRef]
- Abd El-Aty, A.M.; Choi, J.H.; Rahman, M.M.; Kim, S.W.; Tosun, A.; Shim, J.H. Residues and contaminants in tea and tea infusions: A review. Food Addit. Contam. Part A 2014, 31, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Evolution and applications of the QuEChERS method. TrAC Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Lozano, A.; Rajski, Ł.; Belmonte-Valles, N.; Uclés, A.; Uclés, S.; Mezcua, M.; Fernández-Alba, A.R. Pesticide analysis in teas and chamomile by liquid chromatography and gas chromatography tandem mass spectrometry using a modified QuEChERS method: Validation and pilot survey in real samples. J. Chromatogr. A. 2012, 1268, 109–122. [Google Scholar] [CrossRef]
- Chun, O.K.; Kang, H.G.; Kim, M.H. Multiresidue method for the determination of pesticides in Korean domestic crops by gas chromatography/mass selective detection. J. AOAC Int. 2003, 86, 823–831. [Google Scholar] [CrossRef]
- Dehghani, R.; Moosavi, S.G.; Esalmi, H.; Mohammadi, M.; Jalali, Z.; Zamini, N. Surveying of pesticides commonly on the markets of Iran in 2009. J. Environ. Prot. 2011, 2, 1113–1117. [Google Scholar] [CrossRef] [Green Version]
- Seenivasan, S.; Muraleedharan, N. Survey on the pesticide residues in tea in south India. Environ. Monit. Assess. 2011, 176, 365–371. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations, Implications of Maximum Residue Levels (MRLs) on Tea Trade. 2015. Available online: https://www.fao.org/3/i4481e/i4481e.pdf (accessed on 20 September 2022).
- Giacomazzi, S.; Cochet, N. Environmental impact of diuron transformation: A review. Chemosphere 2004, 56, 1021–1032. [Google Scholar] [CrossRef]
- Woollen, B.H.; Marsh, J.R.; Laird, W.J.D.; Lesser, J.E. The metabolism of cypermethrin in man: Differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica 1992, 22, 983–991. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Kumar, S. Hepato-toxic effect of diuron in albino rats. Indian J. Exp. Biol. 1999, 37, 503–504. [Google Scholar] [PubMed]
- Giray, B.; Gurbay, A.; Hincal, F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by Vitamin E or allopurinol. Toxicol. Lett. 2001, 118, 139–146. [Google Scholar] [CrossRef]
- Shukla, Y.; Yadav, A.; Arora, A. Carcinogenic and cocarcinogenic potential of cypermethrin on mouse skin. Cancer Lett. 2002, 182, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Green, T.; Toghill, A.; Lee, R.; Waechter, F.; Weber, E.; Noakes, J. Thiamethoxam induced mouse liver tumors and their relevance to humans Part 1: Mode of action studies in the mouse. Toxicol. Sci. 2005, 86, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastoor, T.; Rose, P.; Lloyd, S.; Peffer, R.; Green, T. Case study: Weight of evidence evaluation of the human health relevance of thiamethoxam-related mouse liver tumors. Toxicol. Sci. 2005, 86, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Richardson, J.R.; Testa, C.M.; Seo, B.B.; Panov, A.V.; Yagi, T.; Matsuno-Yagi, A.; Miller, G.W.; Greenamyre, J.T. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 2007, 100, 1469–1479. [Google Scholar] [CrossRef]
- Imamura, T.; Yanagawa, Y.; Nishikawa, K.; Matsumoto, N.; Sakamoto, T. Two cases of acute poisoning with acetamiprid in humans. Clin. Toxicol. 2010, 48, 851–853. [Google Scholar] [CrossRef]
- Cavas, T.; Cinkilic, N.; Vatan, O.; Yilmaz, D. Effects of fullerenol nanoparticles on acetamiprid induced cytotoxicity and genotoxicity in cultured human lung fibroblasts. Pestic. Biochem. Physiol. 2014, 114, 1–7. [Google Scholar] [CrossRef]
- Bal, R.; Turk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Gundogdu, R.; Gur, S.; Agca, A.; Ulas, M.; Cambay, Z.; et al. Assessment of imidacloprid toxicity on reproductive organ system of adult male rats. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 434–444. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abuzead, S.M.M.; Halawa, S.M. Protective Role of Spirulina platensis against Acute Deltamethrin-Induced Toxicity in Rats. PLoS ONE 2013, 8, e72991. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Daim, M.; El-Bialy, B.E.; Rahman, H.G.A.; Radi, A.M.; Hefny, H.A.; Hassan, A.M. Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: Biochemical and histopathological studies. Biomed. Pharmacother. 2016, 77, 79–85. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takim, K.; Aydemir, M.E. GC-MS and LC-MS Pesticide Analysis of Black Teas Originating from Sri Lanka, Iran, Turkey, and India. Toxics 2023, 11, 34. https://doi.org/10.3390/toxics11010034
Takim K, Aydemir ME. GC-MS and LC-MS Pesticide Analysis of Black Teas Originating from Sri Lanka, Iran, Turkey, and India. Toxics. 2023; 11(1):34. https://doi.org/10.3390/toxics11010034
Chicago/Turabian StyleTakim, Kasim, and Mehmet Emin Aydemir. 2023. "GC-MS and LC-MS Pesticide Analysis of Black Teas Originating from Sri Lanka, Iran, Turkey, and India" Toxics 11, no. 1: 34. https://doi.org/10.3390/toxics11010034