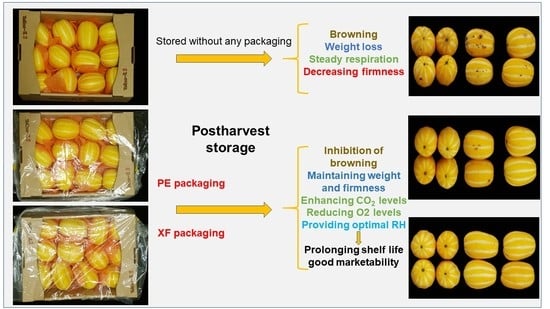
Modified Atmosphere and Humidity Film Reduces Browning Susceptibility of Oriental Melon Suture Tissue during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. In-Package Temperature, Humidity, and Headspace Gas Composition
2.3. Weight Loss of Oriental Melon Fruit
2.4. Firmness Analysis
2.5. Total Soluble Solids
2.6. Surface Color Analysis
2.7. Determination of Browning Injury Index and Marketability
2.8. Light and Scanning Electron Microscopy for Tissue Structure Analysis
2.9. Quantification and Composition of Epicuticular Wax
2.10. Statistical Analysis
3. Results and Discussion
3.1. O2 and CO2 Concentrations
3.2. RH and Weight Loss
3.3. Fruit Quality: Firmness, Total Soluble Solids (TSS), and Surface Color
3.4. Browning of the Fruit Suture and Tissue Structure
3.5. Epicuticular Wax and Specific Water Loss in Oriental Melon Sutures
3.6. Marketability Change by Modified Atmosphere/Modified Humidity Packeging (MAP/MHP)
3.7. Correlation between Modified Atmospherepakaging (MAP) and Cold Injuries (CI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.W.; Qi, H.Y.; Xu, B.H.; Li, Y.; Bin Tian, X.; Jiang, Y.Y.; Xu, X.F.; Lv, D.Q. Ethanol treatment inhibits internal ethylene concentrations and enhances ethyl ester production during storage of oriental sweet melons (Cucumis melo var. makuwa Makino). Postharvest Biol. Technol. 2012, 67, 75–83. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Chung, D.; Lee, Y. Current research status of postharvest and packaging technology of oriental melon (Cucumis melo var. makuwa) in Korea. Korean J. Hortic. Sci. Technol. 2010, 28, 902–911. [Google Scholar]
- Liu, L.; Kakihara, F.; Kato, M. Characterization of six varieties of Cucumis melo L. based on morphological and physiological characters, including shelf-life of fruit. Euphytica 2004, 135, 305. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Nicolai, B.; Lammertyn, J.; Bueso, M.C.; Monforte, A.J.; Fernández-Trujillo, J.P. Aroma volatiles associated with the senescence of climacteric or non-climacteric melon fruit. Postharvest Biol. Technol. 2009, 52, 146–155. [Google Scholar] [CrossRef]
- Yang, D.; Bi, Y.; Chen, X.; Ge, Y.; Zhao, J. Biological control of postharvest diseases with Bacillus subtilis (B1 strain) on muskmelons (Cucumis melo L. cv. Yindi). In Proceedings of the IV International Conference on Managing Quality in Chains-The Integrated View on Fruits and Vegetables Quality 712, Bangkok, Thailand, 30 June 2006; pp. 735–740. [Google Scholar] [CrossRef]
- Choi, J.-W.; Chang, M.-S.; Lee, J.H.; Hong, Y.; Kim, J.G. Changes in Quality of Oriental Melon ‘Smartkkul’ During Vessel Transportation. Korean J. Hortic. Sci. Technol. 2018, 36, 560–568. [Google Scholar]
- Kang, H.-M.; Park, K.-W.; Kim, I.S. Effects of postharvest heat treatment on alleviation chilling injury and improvement storability of oriental melon. Prot. Hortic. Plant Fact. 2005, 14, 137–143. [Google Scholar]
- Lim, B.; Hong, S.; Oh, S.; Chung, D.; Kim, K. Effect of storage temperature on chilling injury and fruit quality of muskmelon. Korean J. Hortic. Sci. Technol. 2010, 28, 248–253. [Google Scholar]
- Jin, Y.Z.; Lv, D.Q.; Liu, W.W.; Qi, H.Y.; Bai, X.H. Ethanol vapor treatment maintains postharvest storage quality and inhibits internal ethylene biosynthesis during storage of oriental sweet melons. Postharvest Biol. Technol. 2013, 86, 372–380. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Cao, S.; Shang, H.; Yang, Z.; Zheng, Y. Methyl jasmonate reduces decay and enhances antioxidant capacity in Chinese bayberries. J. Agric. Food Chem. 2009, 57, 5809–5815. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Amaro, A.L.; Beaulieu, J.C.; Grimm, C.C.; Stein, R.E.; Almeida, D.P. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012, 130, 49–57. [Google Scholar] [CrossRef]
- Henriod, R. Postharvest characteristics of navel oranges following high humidity and low temperature storage and transport. Postharvest Biol. Technol. 2006, 42, 57–64. [Google Scholar] [CrossRef]
- Rodov, V.; Copel, A.; Aharoni, N.; Aharoni, Y.; Wiseblum, A.; Horev, B.; Vinokur, Y. Nested modified-atmosphere packages maintain quality of trimmed sweet corn during cold storage and the shelf life period. Postharvest Biol. Technol. 2000, 18, 259–266. [Google Scholar] [CrossRef]
- Padilla-Zakour, O.; Tandon, K.; Wargo, J. Quality of Modified Atmosphere PackagedHedelfingen’andLapins’ Sweet Cherries. HortTechnology 2004, 14, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Rodov, V.; Horev, B.; Vinokur, Y.; Copel, A.; Aharoni, Y.; Aharoni, N. Modified-atmosphere packaging improves keeping quality of Charentais-type melons. HortScience 2002, 37, 950–953. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of modified (MAP) and controlled atmosphere (CA) storage on the quality and bioactive compounds of blue honeysuckle fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Hafeez, O.; Malik, A.; Khalid, M.; Amin, M.; Khalid, S.; Umar, M. Effect of Modified Atmosphere Packaging on Postharvest Quality of Mango cvs. Sindhri and Sufaid Chaunsa during Storage. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Porat, R.; Weiss, B.; Cohen, L.; Daus, A.; Aharoni, N. Reduction of postharvest rind disorders in citrus fruit by modified atmosphere packaging. Postharvest Biol. Technol. 2004, 33, 35–43. [Google Scholar] [CrossRef]
- Aharoni, N.; Rodov, V.; Fallik, E.; Porat, R.; Pesis, E.; Lurie, S. Controlling Humidity Improves Efficacy of Modified Atmosphere Packaging of Fruits and Vegetables. Acta Hortic. 2008, 8, 121–128. [Google Scholar] [CrossRef]
- Park, M.-H.; Sangwanangkul, P.; Choi, J.-W. Reduced chilling injury and delayed fruit ripening in tomatoes with modified atmosphere and humidity packaging. Sci. Hortic. 2018, 231, 66–72. [Google Scholar] [CrossRef]
- Pesis, E.; Aharoni, D.; Aharon, Z.; Ben-Arie, R.; Aharoni, N.; Fuchs, Y. Modified atmosphere and modified humidity packaging alleviates chilling injury symptoms in mango fruit. Postharvest Biol. Technol. 2000, 19, 93–101. [Google Scholar] [CrossRef]
- Clement, C.; Burrus, M.; Audran, J.-C. Floral organ growth and carbohydrate content during pollen development in Lilium. Am. J. Bot. 1996, 83, 459–469. [Google Scholar] [CrossRef]
- Farber, J.M. Microbiological aspects of modified-atmosphere packaging technology—A Review (1). J. Food Prot. 1991, 54, 58–70. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, S.; Piga, A.; Agabbio, M.; McCollum, T. Film wrapping delays ageing of ‘Minneola’ tangelos under shelf-life conditions. Postharvest Biol. Technol. 1998, 14, 107–116. [Google Scholar] [CrossRef]
- Yehoshua, S.B.; Peretz, J.; Moran, R.; Lavie, B.; Kim, J. Reducing the incidence of superficial flavedo necrosis (noxan) of ‘Shamouti’oranges (Citrus sinensis, Osbeck). Postharvest Biol. Technol. 2001, 22, 19–27. [Google Scholar] [CrossRef]
- Akbudak, B.; Akbudak, N.; Seniz, V.; Eris, A. Effect of pre-harvest harpin and modified atmosphere packaging on quality of cherry tomato cultivars “Alona” and “Cluster”. Br. Food J. 2012, 14, 180–196. [Google Scholar] [CrossRef]
- Rocha, A.M.C.N.; Barreiro, M.G.; Morais, A.M.M.B. Modified atmosphere package for apple ‘Bravo de Esmolfe’. Food Control 2004, 15, 61–64. [Google Scholar] [CrossRef]
- Yang, Q.; Rao, J.; Yi, S.; Meng, K.; Wu, J.; Hou, Y. Antioxidant enzyme activity and chilling injury during low-temperature storage of Kiwifruit cv. Hongyang exposed to gradual postharvest cooling. Hortic. Environ. Biotechnol. 2013, 53, 505–512. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.; Dong, Y.; Szymanski, J.; Lashbrooke, J.; Meir, S.; Almekias-Siegl, E.; Zeisler-Diehl, V.V.; Schreiber, L.; Aharoni, A. A Multilevel Study of Melon Fruit Reticulation Provides Insight into Skin Ligno-Suberization Hallmarks. Plant Physiol. 2019, 179, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Mamrutha, H.M.; Mogili, T.; Jhansi Lakshmi, K.; Rama, N.; Kosma, D.; Udaya Kumar, M.; Jenks, M.A.; Nataraja, K.N. Leaf cuticular wax amount and crystal morphology regulate post-harvest water loss in mulberry (Morus species). Plant Physiol. Biochem. 2010, 48, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Alkalai-Tuvia, S.; Perzelan, Y.; Bosland, P.; Bebeli, P.J.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and water loss in a diverse collection of pepper (Capsicum). Physiol. Plant 2013, 149, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Shen, C.; Farnham, M.W.; Ku, K.-M. Three-dimensional epicuticular wax on plant surface reduces attachment and survival rate of Salmonella during storage. Postharvest Biol. Technol. 2020, 166, 111197. [Google Scholar] [CrossRef]
- Ku, K.M.; Chiu, Y.-C.; Shen, C.; Jenks, M. Leaf cuticular waxes of lettuce are associated with reduced attachment of the foodborne pathogen Salmonella spp. at harvest and after postharvest storage. LWT 2019, 117, 108657. [Google Scholar] [CrossRef]
- Sturm, S.; Schneider, P.; Seger, C.; Stuppner, H. Analysis of Citrullus colocynthis cucurbitacine derivatives with HPLC-SPE-NMR. Sci. Pharm. 2009, 77, 254. [Google Scholar] [CrossRef]
- Adam, S.E.I.; Al-Yahya, M.A.; Al-Farhan, A.H. Response of Najdi sheep to oral administration of Citrullus colocynthis fruits, Nerium oleander leaves or their mixture. Small Rumin. Res. 2001, 40, 239–244. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Lin, H.; Sun, J.; Lin, Y.; Lin, M. Phomopsis longanae Chi-Induced Change in ROS Metabolism and Its Relation to Pericarp Browning and Disease Development of Harvested Longan Fruit. Front. Microbiol. 2018, 9, 2466. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.; Lester, G.; Pennell, K.; Toivonen, P. Oxidative Stress: Importance for Postharvest Quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Tuan, P.A.; Lee, J.; Park, C.H.; Kim, J.K.; Noh, Y.-H.; Kim, Y.B.; Kim, H.; Park, S.U. Carotenoid Biosynthesis in Oriental Melon (Cucumis melo L. var. makuwa). Foods 2019, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lado, J.; Cronje, P.; Rodrigo, M.; Zacarías, L. Resistance to Chilling Injury in Red, Lycopene-Accumulating Tissue of Cold-Stored Grapefruits. Acta Hortic. 2015, 1079, 249–255. [Google Scholar] [CrossRef]









| Storage Time (Days) | Storage Temperature (°C) | Treatment | Firmness (N) | TSS | Hue of Suture |
|---|---|---|---|---|---|
| 0 | 4 | Control | 19.45 ± 0.62 a | 11.60 ± 0.10 a | 96.70 ± 0.33 a |
| PE | 19.45 ± 0.62 a | 11.60 ± 0.10 a | 96.70 ± 0.33 a | ||
| XF | 19.45 ± 0.62 a | 11.60 ± 0.10 a | 96.70 ± 0.33 a | ||
| 0 | 10 | Control | 19.45 ± 0.62 a | 11.60 ± 0.10 a | 96.70 ± 0.33 a |
| PE | 19.45 ± 0.62 a | 11.60 ± 0.10 a | 96.70 ± 0.33 a | ||
| XF | 19.45 ± 0.62 a | 11.60 ± 0.10 a | 96.70 ± 0.33 a | ||
| 7 | 4 | Control | 14.01 ± 0.45 a | 12.17 ± 0.09 b,c,d | 94.77 ± 0.36 a |
| PE | 18.54 ± 0.37 b,c | 12.27 ± 0.13 d,e | 95.57 ± 0.40 a | ||
| XF | 16.57 ± 0.56 b | 12.77 ± 0.03 e | 95.80 ± 0.32 a | ||
| 7 | 10 | Control | 18.93 ± 0.37 c | 11.60 ± 0.15 a | 94.92 ± 0.55 a |
| PE | 19.26 ± 0.34 c | 11.70 ± 0.15 a,bc | 95.62 ± 0.46 a | ||
| XF | 19.86 ± 0.82 c | 11.70 ± 0.07 ab | 95.79 ± 0.34 a | ||
| 14 | 4 | Control | 19.52 ± 0.41 b | 11.60 ± 0.06 a,b | 91.35 ± 0.75 a |
| PE | 18.06 ± 0.74 a,b | 11.27 ± 0.09 a | 95.60 ± 0.52 b | ||
| XF | 19.09 ± 0.45 b | 11.73 ± 0.12 a,b,c | 94.35 ± 0.42 b | ||
| 14 | 10 | Control | 17.67 ± 0.50 a,b | 12.20 ± 0.03 c | 91.66 ± 1.04 a |
| PE | 16.61 ± 0.52 a | 11.70 ± 0.17 a,b,c | 93.60 ± 0.36 a,b | ||
| XF | 17.82 ± 0.33 a,b | 12.00 ± 0.09 b,c | 94.76 ± 0.42 b | ||
| 14 + 2 | 4 | Control | 14.00 ± 0.46 a | 12.03 ± 0.09 b | 88.22 ± 0.62 a |
| PE | 16.74 ± 0.44 a,b,c,d | 12.17 ± 0.03 b | 92.71 ± 0.66 b | ||
| XF | 14.46 ± 0.59 a,b | 11.23 ± 0.13 a | 91.90 ± 0.83 b | ||
| 14 + 2 | 10 | Control | 15.00 ± 0.37 a,b,c | 13.30 ± 0.06 c | 92.70 ± 0.79 b |
| PE | 17.93 ± 0.43 d | 11.00 ± 0.06 a | 93.77 ± 0.46 b | ||
| XF | 17.01 ± 0.69 c,d | 12.50 ± 0.13 b | 92.88 ± 0.60 b | ||
| 14 + 5 | 4 | Control | 14.86 ± 0.65 a | 12.00 ± 0.40 b | 88.32 ± 1.13 a |
| PE | 16.74 ± 0.31 a,b | 11.27 ± 0.15 a,b | 92.06 ± 0.56 b,c | ||
| XF | 16.35 ± 0.55 a,b | 11.07 ± 0.18 a | 90.83 ± 0.88 ab | ||
| 14 + 5 | 10 | Control | 16.22 ± 0.32 a | 11.20 ± 0.13 a,b | 91.98 ± 0.70 b,c |
| PE | 18.22 ± 0.53 b | 10.40 ± 0.03 a | 94.69 ± 0.38 c | ||
| XF | 15.05 ± 0.46 a | 12.00 ± 0.09 b | 94.40 ± 0.37 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-H.; Chang, E.-H.; Yang, H.-J.; Lee, J.-S.; Do, G.-R.; Song, H.J.; Chang, M.-S.; Ku, K.-M. Modified Atmosphere and Humidity Film Reduces Browning Susceptibility of Oriental Melon Suture Tissue during Cold Storage. Foods 2020, 9, 1329. https://doi.org/10.3390/foods9091329
Park M-H, Chang E-H, Yang H-J, Lee J-S, Do G-R, Song HJ, Chang M-S, Ku K-M. Modified Atmosphere and Humidity Film Reduces Browning Susceptibility of Oriental Melon Suture Tissue during Cold Storage. Foods. 2020; 9(9):1329. https://doi.org/10.3390/foods9091329
Chicago/Turabian StylePark, Me-Hea, Eun-Ha Chang, Hae-Jo Yang, Jung-Soo Lee, Gyung-Ran Do, Hyun Jong Song, Min-Sun Chang, and Kang-Mo Ku. 2020. "Modified Atmosphere and Humidity Film Reduces Browning Susceptibility of Oriental Melon Suture Tissue during Cold Storage" Foods 9, no. 9: 1329. https://doi.org/10.3390/foods9091329