Effect of Organic Fertilizers on Selected Health Beneficial Bioactive Compounds and Aroma Profile of Red Topepo Sweet Pepper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organic Fertilizers
2.2. Red Topepo Cultivation and Experimental Design
2.3. Sample Preparation
2.4. Preparation of Ethanol and Water Extracts
2.5. Total Soluble Proteins
2.6. Total Available Carbohydrates
2.7. Total Water Soluble Phenols, Ascorbic Acid, Total Carotenoids, Total Flavonoids, and Vitamin E
2.8. Mineral Assay
2.9. Antioxidant Activities
2.10. Analysis of Volatile Compounds
2.11. Ultra-Fast Gas Chromatography Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Minerals, Primary and Secondary Metabolites of Red Topepo Fruits
3.2. Antioxidant Activities
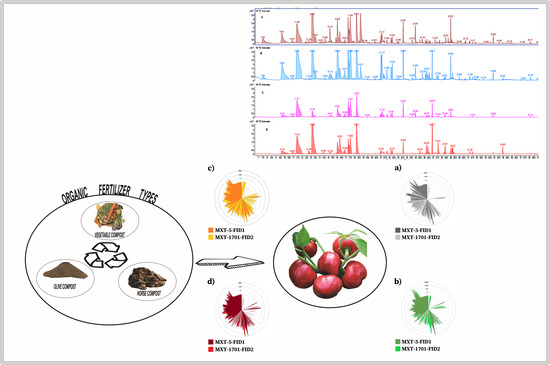
3.3. Aroma Profiling
3.4. Data Fusion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nkansah, G.O.; Norman, J.C.; Martey, A. Sweet pepper is the world’s second most important vegetable after tomato. J. Hortic. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Ríos, A.K.; Medina-Juarez, L.A.; González-Aguilar, G.A.; Gamez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Oboh, G.; Rocha, T.B.J. Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens). J. Food Biochem. 2007, 31, 456–473. [Google Scholar] [CrossRef]
- Habibi, A.; Heidari, G.; Sohrabia, Y.; Badakhshan, H.; Mohammadi, K. Influence of bio, organic and chemical fertilizers on medicinal pumpkin traits. J. Med. Plant. Res. 2011, 523, 5590–5597. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Young, J.W.; Mau, J.L.; Ko, P.T.; Huang, L.C. Antioxidant properties of fermented soybean broth. Food Chem. 2000, 71, 249–254. [Google Scholar] [CrossRef]
- Vågen, I.M.; Aamlid, T.S.; Skjelvåg, A.O. Nitrogen fertilization to broccoli cultivars at different planting times: Yield and nitro-gen use. Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 57, 35–44. [Google Scholar] [CrossRef]
- Akiyama, T.; Shimo, Y.; Yanai, H.; Qin, J.; Ohshima, D.; Maruyama, Y.; Asaumi, Y.; Kitazawa, J.; Takayanagi, H.; Penninger, J.M.; et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 2008, 29, 423–437. [Google Scholar]
- Rekani, O.; Ameen, H.; Ahmed, S.M.R. Effect of different potting mixes on germination and vegetative growth of sweet pepper plant (Capsicum annum L.) under greenhouse conditions. Sci. J. Univ. Zakho 2017, 4, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Huez, M.; Ulery, A.; Samani, Z.; Picchioni, G.; Flynn, R. Response of chile pepper (Capsicum annuum L.) to salt stress and organic and inorganic nitrogen sources: I. growth and yield. Trop. Subtrop. Agroecosys. 2011, 14, 137–147. [Google Scholar]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Jeske-Kaczanowska, A. Are raw materials or composting conditions and time that most influence the maturity and/or quality of composts? Comparison of obtained composts on soil properties. J. Clean. Prod. 2018, 195, 93–101. [Google Scholar] [CrossRef]
- Muscolo, A.; Papalia, T.; Settineri, G.; Romeo, F.; Mallamaci, C. Three different methods for turning olive pomace in resource: Benefits of the end products for agricultural purpose. Sci. Total Environ. 2019, 662, 1–7. [Google Scholar] [CrossRef]
- FAO-UNESCO. World Soil Map, Revised Legend. 1990. Available online: http://www.fao.org/3/as360e/as360e.pdf (accessed on 15 September 2019).
- Kang, M.-C.; Kim, S.-Y.; Kim, E.-A.; Lee, J.-H.; Kim, Y.-S.; Yu, S.-K.; Chae, J.B.; Choe, I.-H.; Cho, J.H.; Jeon, Y.-J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Calderao, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Soil salinity improves nutritional and health promoting compounds in three varieties of lentil (Lens culinaris Med.). Food Biosci. 2020, 35, 100571. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, M.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Davies, S.H.R.; Masten, S.J. Spectrophotometric method for ascorbic acid using dichlo-rophenolindophenol: Elimination of the interference due to iron. Anal. Chim. Acta 1991, 248, 225–227. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef]
- Anonymous. Recommended Practice for Chemical Analysis by Ion Chromatography. Australian Standard AS 3741, Sidney. 1990. Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/As/as3000/3700/3741.pdf (accessed on 15 September 2019).
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C.A.; Lester, P. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying the 2,2′-azobis(3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Methods Enzymol. 1999, 299, 379–389. [Google Scholar]
- Dorman, H.J.D.; Kosar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Ayman, N.; Saber, N.; Haifeng, Z.; Min, Y. Optimization and validation of headspace solid-phase microextraction method coupled with gas chromatography–triple quadrupole tandem mass spectrometry for simultaneous determination of volatile and semi-volatile organic compounds in coking wastewater treatment plant. Environ. Monit. Assess. 2019, 191, 411. [Google Scholar]
- Bogusz, S.; Marchi, T.A.; Teixeira, F.J.; Alcaraz, Z.C.; Teixeira, G.H. Analysis of the volatile compounds chilli peppers (Capsicum spp.) at two stages of maturity by solid phase microextraction and gas chromatography-mass spectrometry. Food Res. Int. 2012, 48, 98–107. [Google Scholar] [CrossRef]
- Rodriguez-Burruezo, A.; Kollmannsberger, H.; Gonzalez-Mas, M.C.; Nitz, S.; Fernando, N. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the annuum chinense frutescens complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef]
- Stierlin, É.; Florence, N.; Xavier, F.; Thomas, M. Development of a headspace solid-phase microextraction gas chromatography-mass spectrometry method to study volatile organic compounds (VOCs) emitted by lavender roots. Chem. Biodivers. 2019, 16. [Google Scholar] [CrossRef]
- Mutarutwa, D.; Navarini, L.; Lonzarich, V.; Compagnone, D.; Pittia, P. GC-MS aroma characterization of vegetable matrices: Focus on 3-alkyl-2-methoxypyrazines. J. Mass Spectrom. 2018, 26. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Last, J.H. Vegetable. In Volatile Compounds in Food and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 203–281. [Google Scholar]
- Houston, M.C.; Karen, J.; Harper, M.S.; Pharm, D. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 2, 3–11. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Fortnightly review: Beneficial effects of potassium. BMJ 2001, 323, 497–501. [Google Scholar] [CrossRef]
- Burnier, M. Should we eat more potassium to better control blood pressure in hypertension? Nephrol. Dial. Transplant. 2019, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaker, J.L.; Deftos, L. Calcium and Phosphate Homeostasis; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; Endotext, MDText.com Inc: South Dartmouth, MA, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK279023/ (accessed on 15 September 2019).
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornelas-Paz, J.D.J.; Martínez-Burrola, J.M.; Ruiz-Cruz, S.; Santana-Rodríguez, V.; Ibarra-Junquera, V.; Olivas, G.I.; Pérez-Martínez, J.D. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010, 119, 1619–1625. [Google Scholar] [CrossRef]
- Gougoulias, N.; Papachatzis, A.; Vagelas, I.; Giurgiulescu, L.; Karaboula, A.; Kalfountzos, D. Total phenols, antioxidant activity and yield, in tomatoes and peppers in a closed greenhouse and comparison with a conventional greenhouse. Stud. UBB Chem. 2016, 61, 295–303. [Google Scholar]
- Oloyede, F.M.; Obisesan, I.O.; Agbaje, G.O.; Obuotor, E.M. Effect of NPK fertilizer on chemical composition of pumpkin (Cucurbita pepo Linn.) seeds. Sci. World J. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sabrina, B.; Djebar, M.-R.; Houria, E.B. Induction of antioxidant enzyme system by a nitrogen fertilizer Npk in wheat Triticum durum. Adv. Environ. Biol. 2012, 6, 85–88. [Google Scholar]
- Omar, N.F.; Hassan, S.A.; Yusoff, U.K.; Abdullah, N.A.; Wahab, P.E.; Sinniah, U. Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 2012, 17, 2378–2387. [Google Scholar] [CrossRef]
- Vignesh, R.; Venkatesh, N.R.; Meenakshisundaram, B.; Jayapradha, R. Novel instant organic fertilizer and analysis of its growth effects on spinach. J. Biol. Sci. 2012, 12, 105–110. [Google Scholar]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content and profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian herb kacip fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.; Vötsch, M.; Vierheilig, H.; Novak, J.; Steinkellner, S.; Zitterl-Eglseer, K.; Franz, C.; Novak, J. Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L.). J. Sci. Food Agric. 2009, 89, 1090–1096. [Google Scholar]
- De Bona, F.D.; Fedoseyenko, D.; von Wirén, N.; Monteiro, F.A. Nitrogen utilization by sulfur-deficient barley plants depends on the nitrogen form. Environ. Exp. Bot. 2011, 74, 237–244. [Google Scholar] [CrossRef]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur Metabolism and Stress Defense Responses in Plants. Trop. Plant. Biol. 2015, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Sereme, A.; Dabire, C.; Koala, M.; Somda, M.K.; Traore, A.F. Influence of organic and mineral fertilizers on the antioxidants and total phenolic compounds level in tomato (solanum lycopersicum) var. mongal f1. J. Exp. Biol. Agric. Sci. 2016, 4, 414–420. [Google Scholar]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Khanduja, K.L. Stable free radical scavenging and antiperoxidative properties of resveratrol in vitro compared with some other bio-flavonoids. Ind. J. Biochem. Biophys. 2003, 40, 416–422. [Google Scholar]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe Vera. Oxid. Med. Cell Long. 2009, 2, 99–106. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Chun, O.K.; Kim, D.-O.; Moon, H.Y.; Kang, H.G.; Lee, C.Y. Contribution of Individual Polyphenolics to Total Antioxidant Capacity of Plums. J. Agric. Food Chem. 2004, 51, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hong, D.L.; Yu, J.W.; Lee, S.M.; Lee, Y.B. Identification of Headspace Volatile Compounds of Blended Coffee and Application to Principal Component Analysis. Prev. Nutr. Food Sci. 2019, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Khodaie, L.; Bamdad, S.; Delazar, A.; Nazemiyeh, H. Antioxidant, total phenol and flavonoid contents of two Pedicularis L. species from eastern Azerbaijan, Iran. BioImpacts 2012, 2, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, F.; Edge, R.; McGarvey, D.J.; Truscott, T.G. Beta-carotene with vitamins E and C offers synergistic cell protection against NOx. FEBS Lett. 1998, 436, 387–390. [Google Scholar]
- Cheetham, P.S.J. Natural sources of flavours. In Food Flavor Technology; Taylor, A.J., Linforth, R.S.T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 127–175. [Google Scholar]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterisation of some volatile constituents of bell peppers. J. Agric. Food Chem. 1969, 17, 1322–1327. [Google Scholar]
- Buttery, R.G.; Seifert, R.M.; Lundin, R.E.; Guadagni, D.G.; Ling, L.C. Characterisation of an important aroma component of bell peppers. Chem. Ind. 1969, 4, 490–491. [Google Scholar]
- Luning, P.A.; Yuksel, D.; Roozen, J.P. Sensory attributes of bell peppers (Capsicum annuum) correlated with composition of volatile compounds. In Proceedings of the 7th Weurman Flavour Research Symposium, Noordwijkerhout, The Netherlands, 15–18 June 1993; pp. 241–248. [Google Scholar]
- Sülsen, V.P.; Lizarraga, E.; Mamadalieva, N.Z.; Lago, J.H.E. Potential of terpenoids and flavonoids from asteraceae as anti-inflammatory, antitumor, and antiparasitic agents. Evid.-Based Complement. Altern. Med. 2017. [Google Scholar] [CrossRef]
- Czernyszewicz, E. The application of principal component analysis to characterize a consumer structure of apple quality. Żywność. Nauka. Technol. Jak. 2008, 2, 119–127. [Google Scholar]
- Kim, D.; Chun, O.; Kim, Y.; Moon, H.; Lee, C. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]






| Treatments | CTR | CO | CV | HD |
|---|---|---|---|---|
| Na+ | 1.25 b ± 0.05 | 0.94 c ± 0.05 | 1.80 a ± 0.10 | 0.85 c ± 0.06 |
| K+ | 17.99 b ± 4.27 | 21.34 b ± 0.14 | 25.21 a ± 1.01 | 23.83 a ± 1.23 |
| Ca2+ | 1.78 c ± 0.04 | 2.00 b ± 0.08 | 2.29 a ± 0.07 | 2.03 b ± 0.08 |
| Mg2+ | 1.31 c ± 0.10 | 2.06 b ± 0.06 | 2.16 b ± 0.06 | 2.48 a ± 0.07 |
| Cl- | 1.14 d ± 0.01 | 2.25 b ± 0.09 | 1.86 c ± 0.03 | 2.55 a ± 0.05 |
| SO42- | 2.40 d ± 0.27 | 4.32 c ± 0.11 | 6.44 a ± 0.15 | 5.39 b ± 0.13 |
| PO43- | 2.62 d ± 0.12 | 3.65 c ± 0.09 | 4.86 a ± 0.06 | 4.49 b ± 0.14 |
| ID | CTR | CO | CV | HD |
|---|---|---|---|---|
| Carbohydrate | 0.64 c ± 0.11 | 1.20 b ± 0.03 | 1.61 a ± 0.11 | 1.18 b ± 0.21 |
| Soluble protein | 631.29 c ± 10.88 | 644.22 bc ± 30.11 | 707.48 b ± 44.42 | 1028.06 a ± 30.08 |
| Total phenols | 451.26 bc ± 53.51 | 405.57 c ± 15.51 | 643.22 a ± 58.05 | 519.46 b ± 16.27 |
| Ascorbic acid | 93.18 b ± 8.99 | 94.90 b ± 15.13 | 159.11 a ± 9.50 | 97.19 b ± 5.73 |
| Carotenoids | 614.67 c ± 13.32 | 637.97 c ± 10.03 | 1582.05 a ± 52.05 | 686.08 b ± 12.01 |
| Vitamin E | 0.63 b ± 0.01 | 0.69 b ± 0.01 | 1.05 a ± 0.18 | 0.90 a ± 0.01 |
| Flavonoids | 2.53 d ± 0.06 | 4.31 c ± 0.14 | 5.21 a ± 0.12 | 4.72 b ± 0.07 |
| ID | CTR | CO | CV | HD |
|---|---|---|---|---|
| DPPH | 60.67 b ± 7.02 | 70.67 b ± 11.46 | 93.33 a ± 5.86 | 84.33 a ± 5.15 |
| FRAP | 606.17 c ± 52.25 | 653.42 c ± 75.55 | 1304.84 a ± 107.97 | 809.49 b ± 168.74 |
| ABTS | 49.33 c ± 2.08 | 49.67 c ± 4.51 | 77.33 a ± 2.15 | 63.3 b ± 1.10 |
| FERROZINE | 25.00 b ± 2.00 | 24.00 d ± 1.00 | 57.00 a ± 3.00 | 14.00 c ± 1.00 |
| COMPOUNDS | RT | CTR | CV | CO | HD |
|---|---|---|---|---|---|
| ALCOHOL (12) | |||||
| 1-pentanol, 4-methyl- | 6.11 | x | x | x | x |
| guaiacol | 14.62 | nd | x | nd | x |
| cis- 3 nonen- 1-ol | 15.35 | x | x | x | x |
| phenylethyl alcohol | 15.65 | nd | nd | nd | x |
| benzyl alcohol | 17.36 | x | x | x | x |
| 1.10-decanediol | 19.15 | x | nd | x | x |
| nona-3.5-dien-2-ol | 20.11 | x | nd | x | nd |
| 4-tert buthylthiophenol | 21.33 | x | x | x | x |
| 1-decanol. 2-ethyl- | 22.43 | x | x | x | x |
| 1-octanol. 2-butyl- | 23.13 | x | x | x | x |
| 1.2-benzenediol. O-(4-ethylbenzoyl)-O’-propargyloxycarbonyl- | 23.55 | nd | x | x | x |
| 1-octanol. 3-butyl- | 31.77 | x | x | x | x |
| 3-hexadecanol | 37.27 | nd | x | x | nd |
| ALDEHYDES (12) | |||||
| hexanal | 4.08 | x | x | x | x |
| benzeneacetaldehyde | 12.91 | x | nd | x | x |
| 2-octenal. (E)- | 13.51 | nd | nd | x | x |
| 4-nonenal. (E)- | 14.96 | x | nd | nd | x |
| 2.6-nonadienal. (E.Z)- | 17.27 | nd | x | x | x |
| 2-nonenal. (Z)- | 17.49 | x | x | x | x |
| Benzaldehyde. 4-ethyl- | 18.05 | nd | nd | nd | x |
| cis-decenal | 18.68 | x | nd | x | x |
| cuminaldeide | 18.90 | x | x | x | nd |
| 7.11-hexadecadienal | 21.14 | x | x | x | x |
| 2.4-decadienal. (E.E)- | 22.24 | x | nd | nd | nd |
| tricyclo[7.1.0.0[1.3]]decane-2-carbaldehyde | 23.00 | x | x | x | x |
| KETONES (2) | |||||
| 4-nonanone | 14.11 | x | x | x | x |
| 2(3H)-furanone. dihydro-5-pentyl- | 24.59 | x | x | x | x |
| ESTERS (9) | |||||
| 4-hexen-1-ol. acetate | 5.65 | x | nd | nd | x |
| phenacylidene diacetate | 9.69 | x | x | x | x |
| cinammilcarbonilate | 14.86 | x | x | nd | x |
| methyl phenylacetate | 16.99 | x | nd | nd | nd |
| myrtenyl acetate | 23.80 | x | x | x | x |
| hexanoic acid. hexyl ester | 25.33 | x | nd | nd | nd |
| 5.8.11.14.17-eicosapentaenoic acid. methyl ester. (all-Z)- | 32.73 | x | nd | x | x |
| oxalic acid. allyl octadecyl ester | 34.55 | x | nd | x | x |
| isopropyl myristate | 37.90 | nd | x | nd | nd |
| HYDROCARBONS (7) | |||||
| 1.3-cyclopentadiene. 5.5-dimethyl-2-ethyl- | 10.85 | x | x | x | xx |
| 1.4-cyclohexadiene. 3-ethenyl-1.2-dimethyl- | 16.03 | x | x | x | x |
| naphthalene. 2-methyl- | 22.16 | x | x | x | x |
| cyclododecane | 25.48 | nd | x | x | x |
| nonane | 30.93 | x | x | x | x |
| 1-octadecyne | 34.95 | nd | x | x | x |
| trans-1.2-diphenylcyclobutane | 35.86 | nd | nd | x | x |
| PYRAZINE (2) | |||||
| pyrazine. 2-methoxy-3-(1-methylpropyl) (2-sec-butyl-3-methoxypyrazine) | 17.92 | x | x | x | x |
| pyrazine. 2-methoxy-3-(2-methylpropyl) (2-isobutyl-3-methoxypyrazine) | 18.21 | x | x | x | x |
| TERPENS (17) | |||||
| citronellal | 12.41 | x | nd | x | x |
| β-ocimene | 12.75 | x | x | x | x |
| 3-Carene | 13.18 | x | x | x | x |
| p-cymene | 14.39 | x | x | x | x |
| carvacrol | 15.08 | x | x | x | x |
| (+)-4-carene | 15.19 | nd | x | x | x |
| cosmene | 15.85 | x | x | x | x |
| trans caren 3-ol | 16.31 | x | x | x | x |
| neo-allo ocimene | 16.75 | x | x | x | x |
| β-ciclocitral | 19.65 | x | nd | x | x |
| perillol | 20.66 | x | x | nd | x |
| cis-verbenol | 21.46 | x | nd | nd | x |
| thymol | 21.69 | x | x | x | x |
| (-)-myrtenol | 22.82 | x | x | x | x |
| geranil linalol | 28.99 | x | x | x | x |
| geraniol | 30.66 | x | x | nd | x |
| trans geranil geraniolo | 31.20 | nd | x | X | x |
| SESQUI TERPENES (19) | |||||
| (E)2, (Z)4, (E)6-allofarnesene | 20.41 | x | x | X | x |
| α-copaene | 24.96 | x | x | X | x |
| β-panasinsene | 25.43 | x | nd | nd | x |
| α-longipinene | 26.23 | x | x | X | x |
| α-santalene | 26.38 | x | x | X | x |
| β-curcumene | 26.88 | x | x | X | x |
| caryophyllene | 27.17 | x | x | nd | nd |
| ylangene | 27.23 | x | x | X | x |
| β-copaene | 27.55 | x | x | X | x |
| santene | 27.64 | x | x | X | x |
| di-epi-α-cedrene | 27.83 | nd | x | nd | nd |
| α-cubebene | 28.15 | x | nd | nd | x |
| nuciferol | 28.25 | x | x | X | x |
| cuparene | 28.34 | x | x | X | x |
| (e)-β-famesene | 28.39 | x | nd | X | x |
| cedrene | 28.72 | x | x | X | nd |
| α-himachalene | 29.12 | x | x | X | x |
| γ-murolene | 29.57 | x | x | nd | x |
| α-ylangene | 30.14 | x | x | nd | nd |
| OTHERS (1) | |||||
| 2 methyl furan | 16.87 | x | x | X | x |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscolo, A.; Papalia, T.; Mallamaci, C.; Carabetta, S.; Di Sanzo, R.; Russo, M. Effect of Organic Fertilizers on Selected Health Beneficial Bioactive Compounds and Aroma Profile of Red Topepo Sweet Pepper. Foods 2020, 9, 1323. https://doi.org/10.3390/foods9091323
Muscolo A, Papalia T, Mallamaci C, Carabetta S, Di Sanzo R, Russo M. Effect of Organic Fertilizers on Selected Health Beneficial Bioactive Compounds and Aroma Profile of Red Topepo Sweet Pepper. Foods. 2020; 9(9):1323. https://doi.org/10.3390/foods9091323
Chicago/Turabian StyleMuscolo, Adele, Teresa Papalia, Carmelo Mallamaci, Sonia Carabetta, Rosa Di Sanzo, and Mariateresa Russo. 2020. "Effect of Organic Fertilizers on Selected Health Beneficial Bioactive Compounds and Aroma Profile of Red Topepo Sweet Pepper" Foods 9, no. 9: 1323. https://doi.org/10.3390/foods9091323