Probiotic Yogurt Fortified with Chickpea Flour: Physico-Chemical Properties and Probiotic Survival during Storage and Simulated Gastrointestinal Transit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Yogurt Fortified with Chickpea Flour
2.2. Analysis of Physicochemical Properties
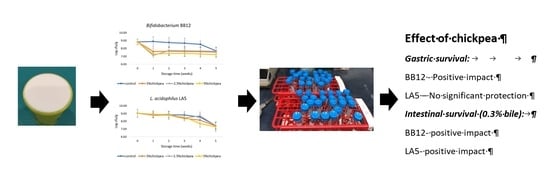
2.3. Microbiological Analysis: Viability of Probiotics and Starter Cultures in Yogurt during Storage
2.4. Preparation of Simulated Gastric and Intestinal Juices
2.5. In Vitro Gastro-Intestinal Tolerance and Probiotic Survival
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Yogurt
3.2. Viability of Probiotics and Yogurt Starters during Storage
3.3. Probiotic Viability during Simulated Gastric and Intestinal Digestion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Araya, M. Guidelines for the Evaluation of Probiotics in Food, Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Khaneghah, A.M.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G. Interactions between Probiotics and Pathogenic Microorganisms in Hosts and Foods: A Review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Boyle, R.J.; Tang, M.L.K. The Role of Probiotics in the Management of Allergic Disease. Clin. Exp. Allergy 2006, 36, 568–576. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Amund, O.D. Exploring the Relationship between Exposure to Technological and Gastrointestinal Stress and Probiotic Functional Properties of Lactobacilli and Bifidobacteria. Can. J. Microbiol. 2016, 62, 715–725. [Google Scholar] [CrossRef]
- Chandan, R.C. History and Consumption Trends. Manuf. Yogurt Fermented Milks 2006, 1, 5400. [Google Scholar]
- Madora, E.P.; Takalani, T.K.; Mashau, M.E. Physicochemical, Microbiological and Sensory Properties of Low Fat Yoghurt Fortified with Carrot Powder. Int. J. Agric. Biol. Eng. 2016, 9, 118–124. [Google Scholar]
- Ghadge, P.N.; Prasad, K.; Kadam, P.S. Effect of Fortification on the Physico-Chemical and Sensory Properties of Buffalo Milk Yoghurt. Electron. J. Environ. Agric. Food Chem. 2008, 7, 2890–2899. [Google Scholar]
- Kumari, A.; Ranadheera, C.S.; Prasanna, P.H.P.; Senevirathne, N.D.; Vidanarachchi, J.K. Development of a Rice Incorporated Synbiotic Yogurt with Low Retrogradation Properties. Int. Food Res. J. 2015, 22, 2032–2040. [Google Scholar]
- Ryan, J.; Hutchings, S.C.; Fang, Z.; Bandara, N.; Gamlath, S.; Ajlouni, S.; Ranadheera, C.S. Microbial, Physico-chemical and Sensory Characteristics of Mango Juice-enriched Probiotic Dairy Drinks. Int. J. Dairy Technol. 2020, 73, 182–190. [Google Scholar] [CrossRef]
- Shihata, A.; Shah, N.P. Influence of Addition of Proteolytic Strains of Lactobacillus delbrueckii Subsp. Bulgaricus to Commercial ABT Starter Cultures on Texture of Yoghurt, Exopolysaccharide Production and Survival of Bacteria. Int. Dairy J. 2002, 12, 765–772. [Google Scholar] [CrossRef]
- Meydani, S.N.; Ha, W.-K. Immunologic Effects of Yogurt. Am. J. Clin. Nutr. 2000, 71, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senok, A.C.; Ismaeel, A.Y.; Botta, G.A. Probiotics: Facts and Myths. Clin. Microbiol. Infect. 2005, 11, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranadheera, C.S.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N. Probiotics in Goat Milk Products: Delivery Capacity and Ability to Improve Sensory Attributes. Compr. Rev. Food Sci. Food Saf. 2019, 18, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus Sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.P.; Lankaputhra, W.E. Improving Viability of Lactobacillus acidophilus and Bifidobacterium spp. in Yogurt. Int. Dairy J. 1997, 7, 349–356. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Ghorbanipour, S.; Mohammadifar, M.A.; Mohammadi, M. Biochemical Properties and Viable Probiotic Population of Yogurt at Different Bacterial Inoculation Rates and Incubation Temperatures. Philipp. Agric. Sci. 2011, 94, 111–116. [Google Scholar]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Zare, F.; Champagne, C.P.; Simpson, B.K.; Orsat, V.; Boye, J.I. Effect of the Addition of Pulse Ingredients to Milk on Acid Production by Probiotic and Yoghurt Starter Cultures. LWT Food Sci. Technol. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of Cow Milk and/or Pea Milk Mixtures by Different Starter Cultures: Physico-Chemical and Sensorial Properties. LWT Food Sci. Technol. 2016, 69, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Raymundo, V.G.; Vélez-Ruiz, J.F. Physicochemical and Rheological Properties of a Dairy Dessert, Enriched with Chickpea Flour. Foods 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.; Awad, S.; El-Sayed, I.; Ibrahim, A. Impact of Chickpea as Prebiotic, Antioxidant and Thickener Agent of Stirred Bio-Yoghurt. Ann. Agric. Sci. 2020, 65, 49–58. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic Viability and Physico-Chemical and Sensory Properties of Plain and Stirred Fruit Yogurts Made from Goat’s Milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.I.; Shah, N.P. Viability of Yoghurt and Probiotic Bacteria in Yoghurts Made from Commercial Starter Cultures. Int. Dairy J. 1997, 7, 31–41. [Google Scholar] [CrossRef]
- Jagannadham, K.; Parimalavalli, R.; Babu, A.S.; Rao, J.S. A Study on comparison between cereal (wheat) and non cereal (chickpea) flour characteristics. Int. J. Curr. Trend Res. 2014, 3, 70–76. [Google Scholar]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as Probiotic Carrier Food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Janer, C.; Peláez, C.; Requena, T. Development of a Fermented Goat’s Milk Containing Probiotic Bacteria. Int. Dairy J. 2003, 13, 827–833. [Google Scholar] [CrossRef]
- Kotz, C.M.; Furne, J.K.; Savaiano, D.A.; Levitt, M.D. Factors Affecting the Ability of a High β-Galactosidase Yogurt to Enhance Lactose Absorption. J. Dairy Sci. 1994, 77, 3538–3544. [Google Scholar] [CrossRef]
- Wu, H.; Hulbert, G.J.; Mount, J.R. Effects of Ultrasound on Milk Homogenization and Fermentation with Yogurt Starter. Innov. Food Sci. Emerg. Technol. 2000, 1, 211–218. [Google Scholar] [CrossRef]
- Keogh, M.K.; O’kennedy, B.T. Rheology of Stirred Yogurt as Affected by Added Milk Fat, Protein and Hydrocolloids. J. Food Sci. 1998, 63, 108–112. [Google Scholar] [CrossRef]
- Noh, H.J.; Seo, H.M.; Lee, J.H.; Chang, Y.H. Physicochemical and Sensory Properties of Yogurt Supplemented with Corni Fructus during Storage. Prev. Nutr. Food Sci. 2013, 18, 45. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.P.; Frasao, B.S.; Silva, A.C.O.; Freitas, M.Q.; Franco, R.M.; Conte-Junior, C.A. Cupuassu (Theobroma Grandiflorum) Pulp, Probiotic, and Prebiotic: Influence on Color, Apparent Viscosity, and Texture of Goat Milk Yogurts. J. Dairy Sci. 2015, 98, 5995–6003. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of Orange Fiber Addition on Yogurt Color during Fermentation and Cold Storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Birollo, G.A.; Reinheimer, J.A.; Vinderola, C.G. Viability of Lactic Acid Microflora in Different Types of Yoghurt. Food Res. Int. 2000, 33, 799–805. [Google Scholar] [CrossRef]
- Tabasco, R.; Paarup, T.; Janer, C.; Peláez, C.; Requena, T. Selective Enumeration and Identification of Mixed Cultures of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. acidophilus, L. paracasei subsp. paracasei and Bifidobacterium lactis in Fermented Milk. Int. Dairy J. 2007, 17, 1107–1114. [Google Scholar]
- Shabala, L.; McMeekin, T.; Budde, B.B.; Siegumfeldt, H. Listeria Innocua and Lactobacillus delbrueckii Subsp. Bulgaricus Employ Different Strategies to Cope with Acid Stress. Int. J. Food Microbiol. 2006, 110, 1–7. [Google Scholar] [CrossRef]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of Lactic Acid Bacteria in the Human Stomach and Adhesion to Intestinal Cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Drakoularakou, A.P.; Kehagias, C.; Karakanas, P.N.; Koulouris, S.; Timbis, D. A Study of the Growth of Lactobacillus acidophilus in Bovine, Ovine and Caprine Milk. Int. J. Dairy Technol. 2003, 56, 59–61. [Google Scholar] [CrossRef]
- Ng, E.W.; Yeung, M.; Tong, P.S. Effects of Yogurt Starter Cultures on the Survival of Lactobacillus acidophilus. Int. J. Food Microbiol. 2011, 145, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Kailasapathy, K.; Rybka, S.L. L. acidophilus and Bifidobacterium spp.—Their Therapeutic Potential and Survival in Yogurt. Aust. J. Dairy Technol. 1997, 52, 28. [Google Scholar]
- Rius, N.; Solé, M.; Francia, A.; Lorén, J.-G. Buffering Capacity and Membrane H+ Conductance of Lactic Acid Bacteria. FEMS Microbiol. Lett. 1994, 120, 291–295. [Google Scholar] [CrossRef]
- De Vuyst, L. Technology Aspects Related to the Application of Functional Starter Cultures. Food Technol. Biotechnol. 2000, 38, 105–112. [Google Scholar]
- Heller, K.J. Probiotic Bacteria in Fermented Foods: Product Characteristics and Starter Organisms. Am. J. Clin. Nutr. 2001, 73, 374s–379s. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, C.G.; Bailo, N.; Reinheimer, J.A. Survival of Probiotic Microflora in Argentinian Yoghurts during Refrigerated Storage. Food Res. Int. 2000, 33, 97–102. [Google Scholar] [CrossRef]
- Güler-Akın, M.B.; Akın, M.S. Effects of Cysteine and Different Incubation Temperatures on the Microflora, Chemical Composition and Sensory Characteristics of Bio-Yogurt Made from Goat’s Milk. Food Chem. 2007, 100, 788–793. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic Potential of Lactobacillus Strains Isolated from Dairy Products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Havenaar, R.; Brink, B.T.; Huis, J.H. Selection of Strains for Probiotic Use. In Probiotics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 209–224. [Google Scholar]
- Sanchez, B.; Ruiz, L.; de Reyes-Gavilan, C.G.L.; Margolles, A. Proteomics of Stress Response in Bifidobacterium. Front. Biosci. 2008, 13, 6905–6919. [Google Scholar] [CrossRef] [Green Version]
- De Boever, P.; Wouters, R.; Verstraete, W. Combined Use of Lactobacillus Reuteri and Soygerm Powder as Food Supplement. Lett. Appl. Microbiol. 2001, 33, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.M.; Pandiella, S.S.; Wang, R.H.; Webb, C. Influence of Malt, Wheat, and Barley Extracts on the Bile Tolerance of Selected Strains of Lactobacilli. Food Microbiol. 2004, 21, 83–89. [Google Scholar] [CrossRef]
- Michida, H.; Tamalampudi, S.; Pandiella, S.S.; Webb, C.; Fukuda, H.; Kondo, A. Effect of Cereal Extracts and Cereal Fiber on Viability of Lactobacillus Plantarum under Gastrointestinal Tract Conditions. Biochem. Eng. J. 2006, 28, 73–78. [Google Scholar] [CrossRef]
- Shimakawa, Y.; Matsubara, S.; Yuki, N.; Ikeda, M.; Ishikawa, F. Evaluation of Bifidobacterium breve Strain Yakult-Fermented Soymilk as a Probiotic Food. Int. J. Food Microbiol. 2003, 81, 131–136. [Google Scholar] [CrossRef]

| Formulation Chickpea Flour | pH | TA (%) | Total Solids (%) | Ash Content (%) | Water Holding Capacity (%) | Viscosity (cP) | Susceptibility to Syneresis (%) |
|---|---|---|---|---|---|---|---|
| 0% | 4.47 ± 0.12 a | 0.78 ± 0.02 a | 12.33 ± 0.35 a | 0.77 ± 0.02 a | 54.00 ± 3.46 a | 3333.33 ± 0.00 a | 49.00 ± 1.73 a |
| 1% | 4.48 ± 0.10 a | 0.78 ± 0.02 a | 12.83 ± 0.01 a | 0.81 ± 0.02 b | 52.66 ± 1.99 a | 5333.33 ± 0.00 b | 45.50 ± 1.51 b |
| 2.5% | 4.46 ± 0.07 a | 0.83 ± 0.03 b | 14.13 ± 0.17 b | 0.93 ± 0.02 c | 57.50 ± 4.99 b | 6499.95 ± 288.22 c | 44.50 ± 1.51 b |
| 5% | 4.44 ± 0.09 a | 0.87 ± 0.03 c | 15.64 ± 0.17 c | 1.09 ± 0.17 d | 58.14 ± 3.08 b | 7499.95 ± 288.22 d | 39.00 ± 1.73 c |
| Formulation Chickpea Flour | Color | ||
|---|---|---|---|
| L * | a * | b * | |
| 0% | 61.35 ± 8.91 a | −0.95 ± 0.19 a | 5.65 ± 4.23 a |
| 1% | 62.60 ± 2.60 a | −1.30 ± 0.56 b | 5.65 ± 4.23 ac |
| 2.5% | 60.4 5± 4.24 a | −1.15 ± 0.61 ab | 6.80 ± 0.69 ab |
| 5% | 62.95 ± 0.09 a | −1.00 ± 0.09 a | 6.45 ± 3.71c |
| Probiotics | Formulation Chickpea Flour | Time (min) during the in Vitro Gastric Digestion | |||
|---|---|---|---|---|---|
| 0 | 1 | 60 | 180 | ||
| Bifidobacterium | 0% | 8.33 ± 0.20 a | 8.72 ± 0.42 a | <1 | <1 |
| 1% | 8.31 ± 0.13 a | 8.54 ± 0.39 a | 4.70 ± 0.00 b | 4.60 ± 0.00 b | |
| 2.5% | 7.54 ± 0.39 a | 8.27 ± 0.17 b | 4.60 ± 0.00 c | 4.30 ± 0.00 d | |
| 5% | 8.20 ± 0.80 a | 8.13 ± 0.03 a | 4.09 ± 0.00 b | <1 | |
| L. acidophilus | 0% | 8.30 ± 0.15 a | 8.12 ± 0.07 a | 5.86 ± 0.25 b | 5.69 ± 0.00 b |
| 1% | 7.85 ± 0.56 a | 8.00 ± 0.01 a | 6.05 ± 0.63 b | 5.62 ± 0.11 b | |
| 2.5% | 8.30 ± 0.03 a | 8.07 ± 0.11 a | 5.86 ± 0.14 b | 4.95 ± 0.00 c | |
| 5% | 8.06 ± 0.03 a | 7.94 ± 0.12 a | 6.02 ± 0.54 b | 5.69 ± 0.00 b | |
| Probiotics | Bile Salt | Formulation Chickpea Flour | Time (min) during the in Vitro Intestinal Digestion | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 60 | 240 | |||
| Bifidobacterium | 0% | 0% | 9.16 ± 0.25 a | 9.12 ± 0.22 a | 4.70 ± 0.00 b | <1 |
| 1% | 8.90 ± 0.16 a | 8.74 ± 0.09 a | 3.00 ± 0.00 b | <1 | ||
| 2.5% | 8.93 ± 0.29 a | 8.95 ± 0.31 a | 5.17 ± 0.00 b | <1 | ||
| 5% | 8.95 ± 0.36 a | 8.84 ± 0.30 a | 4.84 ± 0.00 b | <1 | ||
| 0.3% | 0% | 9.06 ± 0.48 a | 8.90 ± 0.39 a | <1 | <1 | |
| 1% | 8.84 ± 0.41 a | 8.92 ± 0.50 a | 5.20 ± 0.00 b | <1 | ||
| 2.5% | 8.48 ± 0.46 a | 8.32 ± 0.26 a | 5.00 ± 0.00 b | <1 | ||
| 5% | 8.74 ± 0.53 a | 8.71 ± 0.55 a | 4.80 ± 0.00 b | <1 | ||
| L. acidophilus | 0% | 0% | 8.78 ± 0.26 a | 8.36 ± 0.16 b | 5.54 ± 0.55 c | 4.77 ± 0.00 d |
| 1% | 8.69 ± 0.29 a | 8.38 ± 0.03 b | 6.14 ± 0.79 c | 4.47 ± 0.00 d | ||
| 2.5% | 8.69 ± 0.25 a | 8.70 ± 0.31 b | 5.62 ± 0.18 b | 4.95 ± 0.00 c | ||
| 5% | 8.52 ± 0.45 a | 8.07 ± 0.01 a | 5.43 ± 0.16 b | 4.60 ± 0.00 c | ||
| 0.3% | 0% | 8.30 ± 0.15 a | 8.07 ± 0.17 a | <1 | <1 | |
| 1% | 7.85 ± 0.56 a | 7.60 ± 0.54 a | 5.13 ± 0.16 b | <1 | ||
| 2.5% | 8.30 ± 0.03 a | 7.94 ± 0.32 a | 5.25 ± 0.07 b | <1 | ||
| 5% | 8.06 ± 0.03 a | 7.53 ± 0.30 a | 4.60 ± 0.00 b | <1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur Sidhu, M.; Lyu, F.; Sharkie, T.P.; Ajlouni, S.; Ranadheera, C.S. Probiotic Yogurt Fortified with Chickpea Flour: Physico-Chemical Properties and Probiotic Survival during Storage and Simulated Gastrointestinal Transit. Foods 2020, 9, 1144. https://doi.org/10.3390/foods9091144
Kaur Sidhu M, Lyu F, Sharkie TP, Ajlouni S, Ranadheera CS. Probiotic Yogurt Fortified with Chickpea Flour: Physico-Chemical Properties and Probiotic Survival during Storage and Simulated Gastrointestinal Transit. Foods. 2020; 9(9):1144. https://doi.org/10.3390/foods9091144
Chicago/Turabian StyleKaur Sidhu, Manwinder, Fengzhi Lyu, Thomas Patrick Sharkie, Said Ajlouni, and Chaminda Senaka Ranadheera. 2020. "Probiotic Yogurt Fortified with Chickpea Flour: Physico-Chemical Properties and Probiotic Survival during Storage and Simulated Gastrointestinal Transit" Foods 9, no. 9: 1144. https://doi.org/10.3390/foods9091144