Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods
Abstract
:1. Introduction
1.1. Astringency
1.2. General Mechanisms of Astringency
Tannins and Other Polyphenols
1.3. Compounds Causing Astringency
1.4. Influence of Astringency on Oral Perception
1.5. Regulatory Factors of Oral Astringency Perception
1.5.1. pH
1.5.2. Temperature
1.5.3. Saliva
1.5.4. Viscosity
1.5.5. Polysaccharides
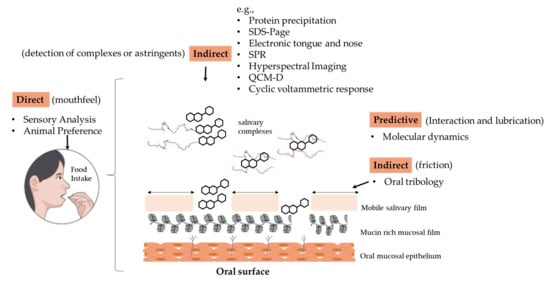
2. Techniques to Quantify Astringency
2.1. Direct Methodologies
2.1.1. Time-Intensity Sensory Evaluation
2.1.2. Descriptive Sensory Analysis
2.1.3. Animal Preference
2.1.4. Ultraviolet Spectroscopy (Indirect Analysis)
2.1.5. SDS-Page Based Method
2.1.6. Protein Precipitation Methods
2.1.7. Electronic Tongue and Nose
2.1.8. Surface Plasmon Resonance and Molecular Imprinted Polymers
2.1.9. Hyperspectral Imaging
2.1.10. One-Component Model Approach
2.1.11. Quartz Crystal Microbalance
2.1.12. Cyclic Voltammetric Response
2.2. Biotribological Assessment
2.3. Simulation by Molecular Dynamics
2.4. Other Techniques
2.4.1. Nuclear Magnetic Resonance
2.4.2. Mass Spectrometry and Synchrotron Radiation
2.4.3. Atomic Force Microscopy
2.4.4. Immunocytochemistry
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Engelen, L.; van der Bilt, A. Oral physiology and texture perception of semisolids. J. Texture Stud. 2007, 39, 83–113. [Google Scholar] [CrossRef]
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and Perception Astringency. Food Sci. Nutr. 2008, 8398. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, N.N.; Matsunami, H. Astringency: A More Stringent Definition. Chem. Senses 2014, 467–469. [Google Scholar] [CrossRef] [Green Version]
- Bate-Smith, E.C. Flavonoid compounds in foods. Adv. Food Res. 1954, 5, 261–300. [Google Scholar]
- Joslyn, M.A.; Goldstein, J.I. Astringency of fruits and fruit products in relation to phenolic content. Adv. Food Res. 1962. [Google Scholar] [CrossRef]
- Lawless, H.T.; Corrigan, C.J.; Lee, C.B. Interactions of astringent substances. Chem. Senses 1994, 19, 141–154. [Google Scholar] [CrossRef]
- Peleg, H.; Bodine, K.K.; Noble, A.C.; Morrisette, C.; Dan, M. The Influence of Acid on Astringency of Alum and Phenolic Compounds. Chem. Senses 1998, 371–378. [Google Scholar] [CrossRef] [Green Version]
- ASTM Standard definitions of terms relating to sensory evaluation of materials and products. In Annual Book of ASTM Standards; ASTM International: Philadelphia, PA, USA, 2004.
- Rossetti, D.; Yakubov, G.E.; Stokes, J.R.; Williamson, A.; Fuller, G.G. Interaction of human whole saliva and astringent dietary compounds investigated by interfacial shear rheology. Food Hydrocoll. 2008, 22, 1068–1078. [Google Scholar] [CrossRef]
- Chen, J.; Stokes, J.R. Rheology and tribology: Two distinctive regimes of food texture sensation. Trends Food Sci. Technol. 2012, 25, 4–12. [Google Scholar] [CrossRef]
- Pradal, C.; Stokes, J.R. Oral tribology: Bridging the gap between physical measurements and sensory experience. Curr. Opin. Food Sci. 2016, 9, 34–41. [Google Scholar] [CrossRef]
- Ranc, H.; Elkhyat, A.; Servais, C.; Mac-Mary, S.; Launay, B.; Humbert, P. Friction coefficient and wettability of oral mucosal tissue: Changes induced by a salivary layer. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 155–161. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Carpenter, G.H. Alternative mechanisms of astringency—What is the role of Saliva? J. Texture Stud. 2013, 44, 364–375. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef] [PubMed]
- De Wijk, R.A.; Prinz, J.F. Mechanisms underlying the role of friction in oral texture. J. Texture Stud. 2006, 37, 413–427. [Google Scholar] [CrossRef]
- Lee, C.A.; Ismail, B.; Vickers, Z.M. The Role of Salivary Proteins in the Mechanism of Astringency. Food Sci. 2012, 77. [Google Scholar] [CrossRef] [PubMed]
- Vardhanabhuti, B.; Kelly, M.A.; Luck, P.J.; Drake, M.A.; Foegeding, E.A. Roles of charge interactions on astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1890–1899. [Google Scholar] [CrossRef] [Green Version]
- Luck, G.; Liao, H.; Murray, N.J.; Grimer, H.R.; Warminski, E.E.; Williamson, M.P.; Haslams, E.; Lilley, T.H. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Pessina, R.; Moio, L. Evaluation of aglianico grape skin and seed polyphenol astringency by SDS–PAGE electrophoresis of salivary proteins after the binding reaction. Food Chem. 2006, 97, 614–620. [Google Scholar] [CrossRef]
- García-Estévez, I.; Ramos-Pineda, A.M.; Escribano, T. Interactions between wine phenolic compounds and human saliva in astringency perception. Food Funct. 2018. [Google Scholar] [CrossRef]
- Bajec, M.R. Astringency and Other Oral Sensations: Biological Sources of Individual Variation and Association with Food and Beverage Behaviour; Brock University: St. Catharines, ON, Canada, 2010. [Google Scholar]
- Rossetti, D.; Bongaerts, J.H.H.; Wantling, E.; Stokes, J.R.; Williamson, A. Food Hydrocolloids Astringency of tea catechins: More than an oral lubrication tactile percept. Food Hydrocoll. 2009, 23, 1984–1992. [Google Scholar] [CrossRef]
- Charlyon, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. Agric. Food Chem. 2002. [Google Scholar] [CrossRef]
- Tournier, C.; Sulmont-rossé, C.; Guichard, E.; Tournier, C.; Sulmont-Rossé, C.; Guichard, E.; Tournier, C.; Elisabeth, C.S. Flavour perception: Aroma, taste and texture interactions. Food 2007, 1, hal-02823959. [Google Scholar]
- Canon, F.; Ballivian, R.; Chirot, F.; Antoine, R.; Sarni-manchado, P.; Dugourd, P.; Lyon, D. Folding of a Salivary Intrinsically Disordered Protein upon Binding to Tannins. J. Am. Chem. Soc. 2011, 7847–7852. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yuyama, N.; Kato, T.; Kawamura, Y. Gustatory responses of cortical neurons in rats. II. Information processing of taste quality. J. Neurophysiol. 1985, 53, 1356–1369. [Google Scholar] [CrossRef]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K. Astringency is a trigeminal sensation that involves the activation of G protein-coupled signaling by phenolic compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [CrossRef]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and flavour perception: Perspectives. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef]
- Prakash, S.; Dan, D.; Tan, Y.; Chen, J. Applications of tribology in studying food oral processing and texture perception. FRIN 2013, 54, 1627–1635. [Google Scholar] [CrossRef]
- Kurogi, M.; Kawai, Y.; Nagatomo, K.; Tateyama, M. Auto-oxidation Products of Epigallocatechin Gallate Activate TRPA1 and TRPV1 in Sensory Neurons. Chem. Senses 2015, 27–46. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, V.; Mateus, N. Protein/Polyphenol Interactions: Past and Present Contributions. Mech. Astringency Percept. 2012, 724–746. [Google Scholar] [CrossRef] [Green Version]
- Boehm, M.W.; Yakubov, G.E.; Stokes, J.R.; Baier, S.K. The role of saliva in oral processing: Reconsidering the breakdown path paradigm. J. Texture Stud. 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T. Salivary Proteins as a Defense Against Dietary Tannins. J. Chem. Ecol. 2006, 1149–1163. [Google Scholar] [CrossRef]
- Mehansho, H.; Clement, S.; Shearesb, B.T.; Smithll, S.; Carlsonl, D.M.; Ross, D.; Grant, P. Induction of Proline-rich Glycoprotein Synthesis in Mouse Salivary Glands by Isoproterenol and by Tannins. J. Boli. Chem. 1985, 260, 4418–4423. [Google Scholar]
- Mehansho, H.; Hagerman, A.N.N.; Clements, S.; Butler, L.; Rogler, J.; Carlson, D.O.N.M. Modulation of proline-rich protein biosynthesis in rat parotid glands by sorghums with high tannin levels. Proc. Natl. Acad. Sci. USA 1983, 80, 3948–3952. [Google Scholar] [CrossRef] [Green Version]
- Valentova, H.; Panovska, Z. SENSORY EVALUATION| Taste. In Encyclopedia of Food Sciences and Nutrition; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Kumari, M.; Jain, S. Tannins: An Antinutrient with Positive Effect to Manage Diabetes. Res. J. Recent Sci. 2015. [Google Scholar]
- Brandão, E.J.F.A. Development of New Formulations Based on Polysaccharides to Reduce the Astringency of Beverages; Universidade do Porto: Porto, Portugal, 2018. [Google Scholar]
- Childs, J.L.; Drake, M. Consumer Perception of Astringency in Clear Acidic Whey Protein Beverages. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef]
- Lee, C.A.; Vickers, Z.M. The astringency of whey protein beverages is caused by their acidity. Int. Dairy J. 2008, 18, 1153–1156. [Google Scholar] [CrossRef]
- Biegler, M.; Delius, J.; Käsdorf, B.T.; Hofmann, T.; Lieleg, O. Cationic astringents alter the tribological and rheological properties of human saliva and salivary mucin solutions. Biotribology 2016, 6, 12–20. [Google Scholar] [CrossRef]
- Lee, C.B.; Lawless, H.T. Time-course of astringent sensations. Chem. Senses 1991, 16, 225–238. [Google Scholar] [CrossRef]
- Guinard, J.; Pangborn, R.M.; Lewis, M.J. Preliminary studies on acidity-astringency interactions in model solutions and wines. J. Sci. Food Agric. 1986, 37, 811–817. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Green, B.G. Oral astringency: A tactile component of flavor. Acta Psychol. 1993, 84, 119–125. [Google Scholar] [CrossRef]
- Laaksonen, O. Astringent Food Compounds and Their Interactions with Taste Properties; University of Turku: Turku, Finland, 2011. [Google Scholar]
- Sano, H.; Egashira, T.; Kinekawa, Y.; Kitabatake, N. Astringency of bovine milk whey protein. J. Dairy Sci. 2005, 88, 2312–2317. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Running, C.A. Dose–response functions and methodological insights for sensory tests with astringent stimuli. J. Sens. Stud. 2018. [Google Scholar] [CrossRef] [Green Version]
- Fleming, E.E.; Ziegler, G.R.; Hayes, J.E. Physiology & Behavior Salivary protein levels as a predictor of perceived astringency in model systems and solid foods. Physiol. Behav. 2016, 163, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Des Gachons, C.; Mura, E.; Speziale, C.; Favreau, C.J.; Dubreuil, G.F.; Breslin, P.A.S. Opponency of astringent and fat sensations. Curr. Biol. 2012, 22, R829–R830. [Google Scholar] [CrossRef] [Green Version]
- Ployon, S.; Brule, M.; Andriot, I.; Morzel, M.; Canon, F. Understanding retention and metabolization of aroma compounds using an in vitro model of oral mucosa. Food Chem. 2020, 126468. [Google Scholar] [CrossRef]
- Lawless, H.T.; Horne, J.; Giasi, P. Astringency of organic acids is related to pH. Chem. Senses 1996, 21, 397–403. [Google Scholar] [CrossRef]
- Siebert, K.J.; Chassy, A.W. An alternate mechanism for the astringent sensation of acids. Food Qual. Prefer. 2004, 15, 13–18. [Google Scholar] [CrossRef]
- Kelly, M.; Vardhanabhuti, B.; Luck, P.; Drake, M.A.; Osborne, J.; Foegeding, E.A. Role of protein concentration and protein-saliva interactions in the astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1900–1909. [Google Scholar] [CrossRef] [Green Version]
- Vardhanabhuti, B.; Foegeding, E. Evidence of interactions between whey proteins and mucin: Their implication on the astringency mechanism of whey proteins at low pH. In Gums and Stabilisers for the Food Industry; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein—Phenolic interactions and associated changes. FRIN 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Peleg, H.; Noble, A.C. Effect of viscosity, temperature and pH on astringency in cranberry juice. Am. J. Enol. Vitic. 1998, 59, 210–214. [Google Scholar] [CrossRef]
- Bajec, M.R.; Pickering, G.J.; De Courville, N. Influence of Stimulus Temperature on Orosensory Perception and Variation with Taste Phenotype. Chemosens. Percept. 2012. [Google Scholar] [CrossRef]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Effect of pH on Astringency in Model Solutions and Wines. J. Agric. Food Chem. 1997, 45, 2211–2216. [Google Scholar] [CrossRef]
- Beecher, J.W.; Drake, M.A.; Luck, P.J.; Foegeding, E.A. Factors regulating astringency of whey protein beverages. J. Dairy Sci. 2008, 91, 2553–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Kato, K. Astringency Removal and Ripening as Related to Temperature during the de-Astringency by Ethanol in Persimmon Fruits. J. Jpn. Soc. Hortic. Sci. 1987, 55, 498–509. [Google Scholar] [CrossRef]
- Ben-aire, R.; Sonego, L. Temperature Affects Astringency Removal and Recurrence in Persimmon. J. Food Sci. 1993, 58, 1397–1400. [Google Scholar] [CrossRef]
- Ross, C.F.; Weller, K. Effect of serving temperature on the sensory attributes of red and white wines. J. Sens. Stud. 2008, 23, 398–416. [Google Scholar] [CrossRef]
- Akyildiz, A.; Aksay, S.; Benli, H.; Kiroǧlu, F.; Fenercioǧlu, H. Determination of changes in some characteristics of persimmon during dehydration at different temperatures. J. Food Eng. 2004, 65, 95–99. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Lamy, E.; Rodrigues, L.; Louro, T.; Capela, F. The Role of Saliva in Food Sensory Perception: Relevant Knowledge to Design Healthy Foods; Formatex Research Center: Badajoz, Spain, 2017. [Google Scholar]
- Prinz, J.F.; De Wijk, R.A.; Huntjens, L. Load dependency of the coefficient of friction of oral mucosa. Food Hydrocoll. 2007, 21, 402–408. [Google Scholar] [CrossRef]
- Kallithraka, S.; Bakker, J.; Clifford, M.N.; Vallis, L. Correlations between saliva protein composition and some T-I parameters of astringency. Food Qual. Prefer. 2001, 12, 145–152. [Google Scholar] [CrossRef]
- Smith, A.K.; June, H.; Noble, C. Effects of viscosity on the Bitterness and Astringency of grape seed tannin. Food Qual. Prefer. 1996, 74, 161–166. [Google Scholar] [CrossRef]
- Courregelongue, S.; Schlich, P.; Noble, A.C. Using repeated ingestion to determine the effect of sweetness, viscosity and oiliness on temporal perception of soymilk astringency. Food Qual. Prefer. 1999, 10, 273–279. [Google Scholar] [CrossRef]
- Ozawa, T.; Lilley, T.H.; Haslam, E. Polyphenol interactions: Astringency and the loss of astringency in ripening fruit. Phytochemistry 1987, 26, 2937–2942. [Google Scholar] [CrossRef]
- Vergara, C.A.O. Efecto de las mezclas de vinos Syrah- viogner y cabernet sauvignon- Syrah sobre la fraccion de polisacaridos y la percepcion de astringencia; Universidad de Chile: Santiago, Chile, 2014. [Google Scholar]
- De Almeida, P.D.V.; Grégio, A.M.T.; Machado, M.Â.N.; de Lima, A.A.S.; Azevedo, L.R. Saliva Composition and Functions: A Comprehensive Review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Çelebioğlu, H.Y.; Gudjónsdóttir, M.; Chronakis, I.S.; Lee, S. Investigation of the interaction between mucins and β-lactoglobulin under tribological stress. Food Hydrocoll. 2016, 54. [Google Scholar] [CrossRef] [Green Version]
- Yakubov, G.E.; Mccoll, J.; Bongaerts, J.H.H.; Ramsden, J.J. Viscous Boundary Lubrication of Hydrophobic Surfaces by Mucin. Langmuir 2009, 2313–2321. [Google Scholar] [CrossRef]
- Jobstl, E.; Connell, J.O.; Fairclough, J.P.A.; Williamson, M.P. Molecular Model for Astringency Produced by Polyphenol/Protein Interactions. Biomacromolecules 2004, 942–949. [Google Scholar] [CrossRef]
- Coles, J.M.; Chang, D.P.; Zauscher, S. Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins. Curr. Opin. Colloid Interface Sci. 2010, 15, 406–416. [Google Scholar] [CrossRef]
- De Wijk, R.A.; Terpstra, M.E.J.; Janssen, A.M.; Prinz, J.F. Perceived creaminess of semi-solid foods. Trends Food Sci. Technol. 2006, 17, 412–422. [Google Scholar] [CrossRef]
- Li, Y.; Joyner, H.S.; Carter, B.G.; Drake, M.A. Effects of fat content, pasteurization method, homogenization pressure, and storage time on the mechanical and sensory properties of bovine milk. J. Dairy Sci. 2018, 2941–2955. [Google Scholar] [CrossRef]
- Malone, M.E.; Appelqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- Troszynska, A.; Narolewska, O.; Robredo, S.; Estrella, I.; Hernández, T.; Lamparski, G.; Amarowicz, R. The effect of polysaccharides on the astringency induced by phenolic compounds. Food Qual. Prefer. 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Mcrae, J.M.; Kennedy, J.A. Wine and Grape Tannin Interactions with Salivary Proteins and Their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, K. Changes in cell wall polysaccharides during fruit ripening. J. Plant Res. 2000, 113, 231–237. [Google Scholar] [CrossRef]
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [Green Version]
- Peryam, D.R.; Pilgrim, F.J. Hedonic scale method of measuring food preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]
- Sjostrom, L.B. The Descriptive Analysis of Flavor. In Food Acceptance Testing Methodology: A Symposium Sponsored by the Quartermaster Food and Container Institute for the Armed Forces, Quartermaster Research and Development Command, US Army Quarte; National Academy of Sciences: Washington, DC, USA, 1954. [Google Scholar]
- Jellinek, G. Introduction to and critical review of modem methods of sensory analysis (odor, taste and flavour evaluation) with special emphasis on descriptive analysis (flavour profile method). J. Nutr. Diet. 1964, 1, 219–260. [Google Scholar]
- Guinard, J.-X.; Pangborn, R.M.; Lewis, M.J. The Time-Course of Astringency in Wine upon Repeated Ingestion. Am. J. Enol. Vitic. 1986, 37, 184–189. [Google Scholar]
- Robichaud, J.L.; Noble, A.C. Astringency and Bitterness of Selected Phenolics in Wine. J. Food Agric. 1990, 343–353. [Google Scholar] [CrossRef]
- Heymann, H.; Lawless, H.T. Sensory Evaluation of Food: Principles and Practices; Springer: New York, NY, USA, 2010. [Google Scholar]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Delarue, J.; Sieffermann, J. Sensory mapping using Flash profile. Comparison with a conventional descriptive method for the evaluation of the flavour of fruit dairy products. Food Qual. Prefer. 2004, 15, 383–392. [Google Scholar] [CrossRef]
- Xue, H.A.N.; Hong, J.; Jun-, L.I.N.; Ying-, Z.; Li, H.A.N.; Xi, X.; Jiao-, J.; Ming, Y.; Ding-, Z.; Base, B.; et al. Quantification of astringency for traditional Chinese medicine based on animal preference index and electronic tongue. China Acad. J. 2017, 42. [Google Scholar] [CrossRef]
- Ramírez, M.; Obreque-slier, E.; Pe, A.; Remigio, O.L. Aversive Effect of Tannic Acid on Drinking Behavior in Mice of an Inbred Strain: Potential Animal Model for Assessing Astringency. J. Agric. Food Chem. 2011, 11744–11751. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jiang, H.; Zhang, D.; Zhang, Y.; Xiong, X.; Jiao, J.; Lin, J. A Novel Quantitative Prediction Approach for Astringency Level of Herbs Based on an Electronic Tongue. Pharmacogn. Mag. 2017, 13, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Power, A.C.; Chapman, J.; Chandra, S.; Cozzolino, D. 6—Ultraviolet-Visible Spectroscopy for Food Quality Analysis; Woodhead Publishing Series: North Rockhampton, Australia, 2019; pp. 91–104. ISBN 9780128142172. [Google Scholar]
- Urbano, M.; de Castro, M.D.L.; Pérez, P.M.; García-Olmo, J.; Gómez-Nieto, M.A. Ultraviolet–visible spectroscopy and pattern recognition methods for differentiation and classification of wines. Food Chem. 2006, 97, 166–175. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P. Leucocyanidol dans les vins rouges. C. R. Acad. Agric. Fr. 1987, 43, 197–199. [Google Scholar]
- Boulet, J.-C.; Ducasse, M.-A.; Cheyner, V. Ultraviolet spectroscopy study of phenolic substances and other major compounds in red wines: Relationship between astringency and the concentration of phenolic substances. Aust. J. Grape Wine Res. 2017, 1–7. [Google Scholar] [CrossRef]
- Boulet, J.; Trarieux, C.; Souquet, J.; Ducasse, M.; Caillé, S.; Samson, A.; Williams, P.; Doco, T.; Cheynier, V. Models based on ultraviolet spectroscopy, polyphenols, oligosaccharides and polysaccharides for prediction of wine astringency. Food Chem. 2016, 190, 357–363. [Google Scholar] [CrossRef]
- Hidayat, B.T.; Wea, A.; Andriati, N. Physicochemical, sensory attributes and protein profile by SDS-PAGE of beef sausage substituted with texturized vegetable protein. Food Res. 2018, 2, 20–31. [Google Scholar] [CrossRef]
- Sarni-manchado, P.; Moutounet, M. Interactions of Grape Seed Tannins with Salivary Proteins. J. Agric. Food Chem. 1999, 42–47. [Google Scholar] [CrossRef]
- Ramos-Pineda, A.M.; Carpenter, G.H.; García-Estévez, I.; Escribano-Bailón, M.T. In fluence of Chemical Species on Polyphenol−Protein Interactions Related to Wine Astringency. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Rinaldi, A.; Gambuti, A.; Moio, L.; Page, S.D.S. Application of the SPI ( Saliva Precipitation Index ) to the evaluation of red wine astringency. Food Chem. 2012, 135, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Ugliano, M.; Rinaldi, A.; Moio, L. Oxygen’s Impact on Red Wine Aging; Wines Vines Analytics: Sonoma, CA, USA, 2016. [Google Scholar]
- Llaudy, M.C.; Canals, R.; Canals, J.-M.; Rozea, N.; Arola, L.; Zamora, F. New Method for Evaluating Astringency in Red Wine. Agric. Food Chem. 2004, 52, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F.; Bjorklund, R.; Ostergren, K. An electronic tongue in the dairy industry. Sens. Actuators B 2005, 112, 299–304. [Google Scholar] [CrossRef]
- Ghasemi-varnamkhasti, M.; Aghbashlo, M. Electronic nose and electronic mucosa as innovative instruments for real-time monitoring of food dryers. Trends Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Zou, G.; Xiao, Y.; Wang, M.; Zhang, H. Detection of bitterness and astringency of green tea with different taste by electronic nose and tongue. PLoS ONE 2018, e0206517. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.M.S.; Sobral, M.M.C.; Delgadillo, I.; Cerdeira, A.; Rudnitskaya, A. Astringency quantification in wine: Comparison of the electronic tongue and FT-MIR spectroscopy. Sens. Actuators B Chem. 2015, 207, 1095–1103. [Google Scholar] [CrossRef]
- Gombau, J.; Nadal, P.; Canela, N.; Gómez-alonso, S.; García-romero, E.; Smith, P. Measurement of the interaction between mucin and oenological tannins by Surface Plasmon Resonance ( SPR ); relationship with astringency. Food Chem. 2019, 275, 397–406. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; Duyne, R.P. Van Localized surface plasmon resonance biosensors. Nanomedicine 2006, 1, 219–228. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Teixeira, N.; De Freitas, V.; Sales, M.G.F.; Sutherland, D.S. A saliva molecular imprinted localized surface plasmon resonance biosensor for wine astringency estimation. Food Chem. 2017, 233, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, F.; Qiu, Z.; Shao, Y. Ripeness Classification of Astringent Persimmon Using Hyperspectral Imaging Technique. Food Bioprocess Technol. 2013. [Google Scholar] [CrossRef]
- Munera, S.; Besada, C.; Blasco, J.; Cubero, S.; Salvador, A.; Talens, P.; Aleixos, N. Astringency assessment of persimmon by hyperspectral imaging. Postharvest Biol. Technol. 2017, 125, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Munera, S.; Aleixos, N.; Besada, C.; Gómez-sanchis, J.; Salvador, A.; Cubero, S. Discrimination of astringent and deastringed hard ‘Rojo Brillante’ persimmon fruit using a sensory threshold by means of hyperspectral imaging. J. Food Eng. 2019, 263, 173–180. [Google Scholar] [CrossRef]
- Lu, L.; Hu, X.; Zhu, Z.; Tian, S.; Chen, Z. One-component Model Approach for Sensing the Sweetness, Sourness and Astringency of Rice. Int. J. Eletrchem. Sci. 2019, 14, 10363–10374. [Google Scholar] [CrossRef]
- Mecea, V.M. From Quartz Crystal Microbalance to Fundamental Principles of Mass Measurements. Anal. Lett. 2007, 2719. [Google Scholar] [CrossRef]
- Vashist, S.K.; Vashist, P. Recent Advances in Quartz Crystal Microbalance-Based Sensors. J. Sens. 2011, 2011. [Google Scholar] [CrossRef]
- Yan, Y.; Hu, J.; Yao, P. Effects of Casein, Ovalbumin, and Dextran on the Astringency of Tea Polyphenols Determined by Quartz Crystal Microbalance with Dissipation. Lungmuir 2009, 397–402. [Google Scholar] [CrossRef]
- Zimmermann, A.R.; Delius, J.; Friedrichs, J.; Stehl, S.; Hofmann, T.; Hannig, C. Impact of oral astringent stimuli on surface charge and morphology of the protein-rich pellicle at the tooth–saliva interphase. Colloids Surf. B Biointerfaces 2018. [Google Scholar] [CrossRef]
- Petrovic, S.C. Correlation of Perceived Wine Astringency to Cyclic Voltammetric Response. Am. J. Enol. Vitic. 2009, 60, 373–378. [Google Scholar]
- Makhotkina, O.; Kilmartin, P. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of total polyphenol content of wines by means of voltammetric techniques: Cyclic voltammetry vs differential pulse voltammetry. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jost, H.P. Tribology—Origin and future. Wear 1990, 136. [Google Scholar] [CrossRef]
- Bhushan, B. Principles and Applications of Tribology; John Wiley & Sons: Colombus, OH, USA, 1999. [Google Scholar]
- Upadhyay, R.; Brossard, N.; Chen, J. Mechanisms underlying astringency: Introduction to an oral tribology approach. J. Phys. D Appl. Phys. 2016, 49, 104003. [Google Scholar] [CrossRef]
- Selway, N.; Stokes, J.R. Soft Materials Deformation, Flow, and Lubrication Between Compliant Substrates: Impact on Flow Behavior, Mouthfeel, Stability, and Flavor. Annu. Rev. Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Sarkar, A.; Andablo-reyes, E.; Bryant, M.; Dowson, D.; Neville, A. ScienceDirect Lubrication of soft oral surfaces. Curr. Opin. Colloid Interface Sci. 2019, 39, 61–75. [Google Scholar] [CrossRef]
- Laguna, L.; Farrell, G.; Bryant, M.; Sarkar, A. Relating rheology and tribology of commercial dairy colloids to sensory perception. R. Soc. Chem. 2016. [Google Scholar] [CrossRef]
- Krzeminski, A.; Wohlhüter, S.; Heyer, P.; Utz, J.; Hinrichs, J. Measurement of lubricating properties in a tribosystem with different surface roughness. Int. Dairy J. 2012, 26. [Google Scholar] [CrossRef]
- Dresselhuis, D.M.; De Hoog, E.H.A.; Stuart, M.A.C.; Aken, G.A. Van Application of oral tissue in tribological measurements in an emulsion perception context. Food Hydrocoll. 2008, 22, 323–335. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Prakash, S. Lubrication studies of fl uid food using a simple experimental set up. Food Hydrocoll. 2014, 6–11. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Gilmore, M.M.; Beauchamp, G.K.; Green, B.G. Psychophysical evidence that oral astringency is a tactile sensation. Chem. Senses 1993, 18, 405–417. [Google Scholar] [CrossRef]
- Brossard, N.; Cai, H.; Osorio, F.; Bordeu, E.; Chen, J. “Oral” Tribological Study on the Astringency Sensation of Red Wines. J. Texture Stud. 2016, 47, 392–402. [Google Scholar] [CrossRef]
- Li, Y.; Lee, A.P.; Drake, M.A. Impact of pasteurization method and fat on milk: Relationships among rheological, tribological, and astringency behaviors. Int. Dairy J. 2017. [Google Scholar] [CrossRef]
- Shewan, H.M.; Pradal, C.; Stokes, J.R. Tribology and its growing use toward the study of food oral processing and sensory perception. J. Text. Stud. 2019, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Vanga, S.K.; Orsat, V.; Raghavan, V. Application of molecular dynamic simulation to study food proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2779–2789. [Google Scholar] [CrossRef]
- De Azevedo, W.F. Molecular Dynamics Simulations of Protein Targets Identified in. Curr. Med. Chem. 2011, 19, 1353–1366. [Google Scholar] [CrossRef]
- Ramos-Pineda, A.M.; García-Estévez, I.; Brás, N.F.; Martín Del Valle, E.M.; Dueñas, M.; Escribano Bailón, M.T. Molecular Approach to the Synergistic Effect on Astringency Elicited by Mixtures of Flavanols. J. Agric. Food Chem. 2017, 65, 6425–6433. [Google Scholar] [CrossRef] [Green Version]
- Schall, J.D.; Mikulski, P.T.; Chateauneuf, G.M.; Gao, G.; Harrison, J.A. Molecular dynamics simulations of tribology. Superlubricity 2007, 79–102. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Yang, S.H.; Chung, G. Functional characterization of naturally occurring wild soybean mutant (sg- 5) lacking astringent saponins using whole genome sequencing approach. Plant Sci. 2017, 267, 148–156. [Google Scholar] [CrossRef]
- Vale, N.; Gomes, P.; Mateus, N.; De Freitas, V.; Heredia, F.J.; Ferrer-Gallego, R.; Hernández-Hierro, J.M. Interaction between Wine Phenolic Acids and Salivary Proteins by Saturation-Transfer Di ff erence Nuclear Magnetic Resonance Spectroscopy (STD-NMR) and Molecular Dynamics Simulations. J. Agric. Food Chem. 2017, 65, 6434–6441. [Google Scholar] [CrossRef]
- Chen, G.; Huang, K.; Miao, M. Molecular Dynamics Simulation for Mechanism Elucidation of Food Processing and Safety: State of the Art. Food Sci. Food Saf. 2019, 18, 243–263. [Google Scholar] [CrossRef] [Green Version]
- Cala, O.; Fabre, S.; Fouquet, E.; Dufourc, E.J.; Pianet, I. Comptes Rendus Chimie NMR of human saliva protein/wine tannin complexes. Towards deciphering astringency with physico-chemical tools. C. R. Chim. 2010, 13, 449–452. [Google Scholar] [CrossRef]
- Canon, F.; Paté, F.; Meudec, E.; Marlin, T.; Cheynier, V.; Giuliani, A.; Sarni-Manchado, P. Characterization, stoichiometry, and stability of salivary protein–tannin complexes by ESI-MS and ESI-MS/MS. Anal. Bioanal. Chem. 2009, 2535–2545. [Google Scholar] [CrossRef]
- Simon, C.; Barathieu, K.; Laguerre, M.; Schmitter, J.; Fouquet, E.; Pianet, I.; Dufourc, E.J. Three-Dimensional Structure and Dynamics of Wine Tannin-Saliva Protein. A Multitechnique Approach. Biochemistry 2003, 10385–10395. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Milosavljević, A.R.; van der Rest, G.; Réfrégiers, M.; Nahon, L.; Sarni-Manchado, P.; Cheynier, V.; Giuliani, A. Photodissociation and Dissociative Photoionization Mass Spectrometry of Proteins and Noncovalent Protein-Ligand Complexes. Angew. Chem. 2013, 8377. [Google Scholar] [CrossRef]
- Davies, H.S.; Pudney, P.D.A.; Georgiades, P.; Waigh, T.A.; Hodson, N.W.; Ridley, C.E.; Blanch, E.W.; Thornton, D.J. Reorganisation of the Salivary Mucin Network by Dietary Components: Insights from Green Tea Polyphenols. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Regulatory Factor | Purpose of the Study | Study Findings | References |
|---|---|---|---|
| pH |
|
| [47] |
|
| [54] | |
|
| [43] | |
|
| [31] | |
|
| [59] | |
|
| [57] | |
|
| [60] | |
|
| [52] | |
|
| [40] | |
| Temperature |
|
| [61] |
|
| [57] | |
|
| [62] | |
|
| [63] | |
|
| [64] | |
|
| [65] | |
| Saliva |
|
| [66] |
|
| [67] | |
|
| [68] | |
|
| [69] | |
|
| [13] | |
| Viscosity |
|
| [57] |
|
| [70] | |
|
| [71] | |
|
| [60] | |
| Polysaccharides |
|
| [38] |
|
| [18] | |
|
| [72] | |
|
| [73] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, M.A.; Pastrana, L.M.; Fuciños, P.; Abreu, C.S.; Oliveira, S.M. Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods. Foods 2020, 9, 1124. https://doi.org/10.3390/foods9081124
Pires MA, Pastrana LM, Fuciños P, Abreu CS, Oliveira SM. Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods. Foods. 2020; 9(8):1124. https://doi.org/10.3390/foods9081124
Chicago/Turabian StylePires, Mariana A., Lorenzo M. Pastrana, Pablo Fuciños, Cristiano S. Abreu, and Sara M. Oliveira. 2020. "Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods" Foods 9, no. 8: 1124. https://doi.org/10.3390/foods9081124