Cuticular Wax Composition of Wild and Cultivated Northern Berries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
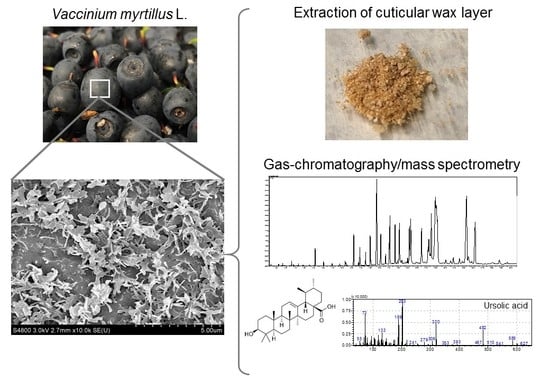
2.2. Extraction of Cuticular Wax
2.3. Analysis Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.4. Data Analysis
3. Results and Discussion
3.1. Wax Amounts
3.2. Compound Classes Found in the Cuticular Wax
3.3. Triterpenoids
3.4. Alkanes
3.5. Fatty Acids
3.6. Principal Components Analysis of Wax Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Compound Class | Substance | RT, Min | Rowanberry | Hawthorn | A. Cranberry | Gaultheria | B. Crowberry | Lingonberry | Bilberry | Bog Bilberry |
|---|---|---|---|---|---|---|---|---|---|---|
| Triterpene | β-sitosterol | 21.17 | 0.291 | 0.39 | 0.315 | 0.3 | ≤LOD | 0.3 | ≤LOD | ≤LOD |
| β-amyrin | 21.38 | ≤LOD | ≤LOD | 0.3 | 0.956 | 0.356 | 0.24 | 0.76 | 0.747 | |
| α-amyrin | 21.83 | 0.3 | 0.3 | 0.25 | 1.958 | 2.255 | 1.072 | 1.89 | ≤LOD | |
| Lupeol | 22.04 | 0.3 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.946 | 0.3 | ≤LOD | |
| Lanosterol | 22.14 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.35 | 2.583 | ≤LOD | ≤LOD | |
| Uvaol | 23.83 | ≤LOD | ≤LOD | 0.445 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.3 | |
| Erythrodiol | 24.43 | 0.314 | 0.03 | ≤LOD | 0.004 | ≤LOD | ≤LOD | 0.06 | ≤LOD | |
| Betulinic acid | 25.13 | 0.232 | ≤LOD | 0.231 | ≤LOD | ≤LOD | 1.326 | 2.811 | 2.072 | |
| Ursolic acid | 25.46 | 2.188 | 2.408 | 6.628 | 4.379 | 4.983 | 4.074 | 2.968 | 3.421 | |
| Ursolic aldehyde | 26.2 | 0.589 | 0.197 | ≤LOD | 0.023 | 0.832 | 0.264 | 0.19 | 0.418 | |
| Betulonic acid | 26.5 | ≤LOD | ≤LOD | 0.013 | 0.184 | 0.309 | ≤LOD | ≤LOD | 1.224 | |
| Subtotal | 4.214 | 3.325 | 8.182 | 7.804 | 9.085 | 10.805 | 8.979 | 8.182 | ||
| Alkane | C20 | 4.37 | 0.05 | 0.286 | ≤LOD | ≤LOD | 0.233 | 0.05 | ≤LOD | 0.05 |
| C23 | 7.34 | ≤LOD | 0.05 | ≤LOD | 0.09 | ≤LOD | ≤LOD | ≤LOD | 0.077 | |
| C25 | 10.03 | 0.057 | 0.057 | 0.057 | 0.065 | 0.276 | 0.057 | ≤LOD | ≤LOD | |
| C27 | 13.24 | 0.152 | 0.151 | 0.107 | 1.515 | 2.268 | 0.05 | 0.282 | 0.07 | |
| C28 | 14.51 | 0.111 | 0.012 | ≤LOD | 0.079 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| C29 | 15.65 | 0.771 | 1.19 | 0.059 | 1.196 | 0.632 | 0.094 | 0.084 | 0.256 | |
| C30 | 16.64 | 0.105 | 0.11 | 0.049 | 0.203 | 0.158 | 0.115 | 0.057 | ≤LOD | |
| C31 | 17.64 | 0.222 | 0.158 | 0.075 | 1.832 | 2.188 | 0.393 | 0.075 | 0.075 | |
| C33 | 19.9 | 0.039 | 0.05 | 0.072 | 0.243 | 0.342 | 0.05 | 0.07 | ≤LOD | |
| Subtotal | 1.507 | 2.064 | 0.419 | 5.223 | 6.097 | 0.809 | 0.568 | 0.528 | ||
| Fatty acid | Palmitic acid | 5.23 | 0.347 | 0.143 | 0.065 | 0.087 | 0.025 | 0.137 | ≤LOD | ≤LOD |
| Stearic acid | 6.74 | ≤LOD | 0.012 | 0.032 | 0.037 | 0.017 | 0.022 | 0.25 | 0.34 | |
| Nonadecanoic acid | 7.81 | 0.052 | 0.06 | 0.02 | 0.013 | 0.018 | 0.038 | 0.5 | ≤LOD | |
| Eicosanoic acid | 9.12 | 0.028 | 0.025 | 0.171 | 0.017 | 0 | 0.027 | ≤LOD | ≤LOD | |
| Behenic acid | 12.4 | 0.077 | 0.119 | 0.182 | 0.092 | 0.647 | 0.199 | 0.397 | 0.272 | |
| Lignoceric acid | 14.93 | ≤LOD | ≤LOD | ≤LOD | 0.16 | 0.3 | 0.414 | 3.107 | 3.035 | |
| Hexacosanoic acid | 17.03 | 0.144 | 0.019 | ≤LOD | 0.148 | 0 | 0.27 | 7.643 | 5.23 | |
| Octacosanoic acid | 18.83 | 0.186 | 0.315 | 0.214 | 0.277 | 0.205 | 0.478 | 1.286 | 0.497 | |
| Nonacosanoic acid | 20.85 | 0.002 | 0.028 | 0.09 | 0.051 | 0.097 | 0.363 | 0.68 | 0.562 | |
| Triacontanoic acid | 21.54 | 0.207 | 0.218 | 1.005 | 0.611 | 0.263 | 0.716 | 0.658 | 1.076 | |
| Subtotal | 1.043 | 0.939 | 1.779 | 1.493 | 1.572 | 2.664 | 14.521 | 11.012 | ||
| Acetate | Hexacosyl acetate | 14.64 | ≤LOD | 0.065 | ≤LOD | ≤LOD | 0.234 | 0.414 | 1.425 | 4.941 |
| Alcohol | Phytol | 6.18 | ≤LOD | 0.056 | ≤LOD | ≤LOD | 0.033 | ≤LOD | 0.003 | ≤LOD |
| Docosanol | 10.93 | 0.344 | 0.694 | 0.007 | 0.348 | 0.391 | 0.221 | 0.171 | 1.253 | |
| Tetracosanol | 13.88 | 0.298 | 0.954 | 0.037 | 2.523 | 0.205 | 0.024 | 0.086 | 0.032 | |
| 9-C27-ol | 15.37 | 0.309 | ≤LOD | ≤LOD | ≤LOD | 1.359 | 1.958 | 0.164 | 0.022 | |
| 1-Hexacosanol | 16.12 | 0.296 | 0.593 | 0.161 | 0.729 | ≤LOD | 0.028 | ≤LOD | 0.738 | |
| 9-C28-ol + 10-C28-ol | 16.37 | 0.169 | 0.014 | 0.041 | ≤LOD | 0.025 | ≤LOD | ≤LOD | 0.053 | |
| 10-C29-ol | 17.27 | 3.704 | 0.07 | 0.026 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| 8,9-C27-diol | 18.56 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.239 | ≤LOD | |
| 11-C31-ol | 19.42 | 0.188 | ≤LOD | 0.004 | 0.065 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| 1-Triacontanol | 20.48 | 0.496 | 0.042 | 0.313 | 0.267 | 0.094 | 0.323 | 0.096 | 0.75 | |
| 1-Dotriacontanol | 23.49 | 0.087 | 0.011 | ≤LOD | 0.418 | ≤LOD | ≤LOD | 0.32 | 1.528 | |
| Subtotal | 5.891 | 2.434 | 0.589 | 4.35 | 2.107 | 2.554 | 1.079 | 4.376 | ||
| Aldehyde | Hexacosanal | 12.33 | 0.045 | 0.126 | 0.049 | 0.058 | 0.047 | 0.177 | 0.048 | 0.008 |
| Heptacosanal | 13.75 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 3.908 | 0.289 | 0.271 | 0.063 | |
| Octacosanal | 14.4 | ≤LOD | ≤LOD | 0.202 | 0.087 | 0.235 | 0.014 | 0.039 | 0.033 | |
| Triacontanal | 17.09 | 0.358 | 0.076 | 1.056 | 0.389 | 0.664 | 0.432 | 1.883 | 5.753 | |
| Dotriacontanal | 19.31 | 0.421 | 0.175 | 2.819 | 0.11 | 0.599 | 3.463 | 2.432 | 0.296 | |
| Subtotal | 0.824 | 0.377 | 4.126 | 0.644 | 5.453 | 4.375 | 4.673 | 6.153 | ||
| Carbohydrate | beta.-D-Allopyranose | 4.85 | ≤LOD | 0.559 | 0.006 | 0.027 | 0.027 | ≤LOD | 0.004 | 0.006 |
| Myo-Inositol | 5.8 | ≤LOD | 0.093 | 0.019 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Carbohydrate | 9.55 | ≤LOD | ≤LOD | 0.036 | 0.01 | 0.023 | 0.024 | 0.099 | 0.112 | |
| Subtotal | 0 | 0.652 | 0.061 | 0.037 | 0.05 | 0.024 | 0.103 | 0.118 | ||
| Sesquiterpene | Abscisic acid | 6.61 | 0.08 | 0.084 | 0.144 | 0.058 | 0.018 | 0.011 | 0.024 | 0.034 |
| Ester | Octacosanoate | 19.17 | 0.186 | 0.315 | 0.214 | 0.277 | 0.205 | 0.478 | 1.286 | 0.497 |
| Ketone | Nonacosane-8,10-dione | 18.65 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.287 | ≤LOD |
| Hentriacontane-10,12-dione | 21.27 | 0.014 | 0.051 | 0.551 | 0.615 | 0.133 | 0.139 | ≤LOD | 0.493 | |
| Subtotal | 0.014 | 0.051 | 0.551 | 0.615 | 0.133 | 0.139 | 0.287 | 0.493 | ||
| Phenolic acid | Syringic acid | 4.3 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD |
| p-Coumaric acid | 4.65 | ≤LOD | ≤LOD | ≤LOD | 0.173 | 0.037 | 0.435 | ≤LOD | 0.021 | |
| Methyl caffeate | 5.11 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.053 | 0.003 | 0.044 | 0.023 | |
| Ferulic acid | 5.65 | 0.016 | 0.013 | 0.018 | 0.199 | 0.009 | ≤LOD | ≤LOD | 0.027 | |
| Subtotal | 0.016 | 0.013 | 0.018 | 0.372 | 0.099 | 0.438 | 0.044 | 0.071 | ||
| Tocopherols | alpha.-Tocopherol | 18.32 | ≤LOD | 0.224 | 0.622 | 0.029 | 0.011 | 0.153 | 0.187 | 0.112 |
| Total | 13.775 | 10.543 | 16.705 | 20.902 | 25.064 | 22.864 | 33.176 | 36.517 |
| Compound Class | Substance | RT,min | Patriot | Polaris | Blue Crop | Chandler | Duke | Blue Gold | Chippewa | North Blue |
|---|---|---|---|---|---|---|---|---|---|---|
| Triterpene | β-sitosterol | 21.17 | ≤LOD | ≤LOD | 0.074 | 0.3 | ≤LOD | ≤LOD | 0.235 | 0.3 |
| β-amyrin | 21.38 | 1.795 | 1.914 | ≤LOD | 1.865 | 3.195 | ≤LOD | 2.576 | 1.439 | |
| α-amyrin | 21.83 | 9.519 | 11.066 | 6.76 | 4.211 | 3.842 | 1.625 | 6.374 | 2.351 | |
| Lupeol | 22.04 | ≤LOD | 10.196 | 9.21 | 1.418 | 1.017 | 11.2 | ≤LOD | ≤LOD | |
| Lanosterol | 22.14 | ≤LOD | ≤LOD | 0.508 | 0.35 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Uvaol | 23.83 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Erythrodiol | 24.43 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Betulinic acid | 25.13 | ≤LOD | 1.994 | 0.244 | 5.065 | 8.33 | 6.695 | 1.964 | 2.165 | |
| Ursolic acid | 25.46 | 4.241 | 9.304 | 2.116 | 2.938 | 3.356 | 3.231 | 0.459 | 5.115 | |
| Ursolic aldehyde | 26.2 | ≤LOD | ≤LOD | ≤LOD | 0.007 | ≤LOD | 0.012 | ≤LOD | ≤LOD | |
| Betulonic acid | 26.5 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Subtotal | 15.555 | 34.474 | 18.912 | 16.154 | 19.74 | 22.763 | 11.608 | 11.37 | ||
| Alkane | C20 | 4.37 | 0.05 | 0.082 | ≤LOD | 0.05 | 0.05 | ≤LOD | 0 | 0.05 |
| C23 | 7.34 | 0.05 | 0.074 | ≤LOD | 0.05 | 0.05 | ≤LOD | 0.05 | ≤LOD | |
| C25 | 10.03 | 0.05 | 0.057 | 0.074 | 0.057 | 0.057 | ≤LOD | 0.057 | 0.057 | |
| C27 | 13.24 | 0.05 | 0.074 | 0.093 | 0.064 | 0.05 | 0.075 | 0.778 | 0.157 | |
| C28 | 14.51 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| C29 | 15.65 | 1.138 | 0.691 | 0.219 | 0.691 | 0.676 | 0.648 | 0.453 | 1.226 | |
| C30 | 16.64 | 0.043 | 0.1 | 0.075 | 0.057 | 0.05 | 0.075 | 0.146 | 0.093 | |
| C31 | 17.64 | 0.937 | 0.758 | 0.075 | 0.423 | 0.138 | 0.075 | 0.08 | 0.596 | |
| C33 | 19.9 | 0.079 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.075 | 0.044 | 0.07 | |
| Subtotal | 2.397 | 1.836 | 0.536 | 1.392 | 1.071 | 0.948 | 1.608 | 2.249 | ||
| Fatty acid | Palmitic acid | 5.23 | 0.4 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 | 0.5 | 0.126 |
| Stearic acid | 6.74 | 0.25 | 0.3 | 0.25 | 0.3 | 0.25 | 0.3 | 0.25 | 0.13 | |
| Nonadecanoic acid | 7.81 | 0.769 | 0.684 | 0.84 | 1.084 | 1.349 | 1.362 | 0.195 | 0.34 | |
| Eicosanoic acid | 9.12 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Behenic acid | 12.4 | 0.806 | 1.304 | 0.34 | 1.213 | 0.457 | 0.307 | 1.809 | 0.74 | |
| Lignoceric acid | 14.93 | 1.076 | 1.013 | 0.98 | 2.466 | 1.106 | 0.87 | 1.738 | ≤LOD | |
| Hexacosanoic acid | 17.03 | 0.241 | 1.266 | 0.336 | 4.402 | 0.833 | 0.671 | 1.7 | 0.607 | |
| Octacosanoic acid | 18.83 | 0.75 | 2.068 | 1.062 | 1.518 | 0.993 | 3.26 | 3.249 | 1.015 | |
| Nonacosanoic acid | 20.85 | 1.549 | 2.205 | 1.2 | 1.861 | 2.197 | 1.56 | 2.687 | 0.9 | |
| Triacontanoic acid | 21.54 | 6.958 | 6.005 | 4 | 1.8 | 3.2 | 3.5 | ≤LOD | 9.558 | |
| Subtotal | 12.799 | 15.145 | 9.408 | 14.944 | 10.785 | 12.13 | 12.128 | 13.416 | ||
| Acetate | Hexacosyl acetate | 14.64 | 0.974 | 1.013 | 1.542 | 1.927 | 1.294 | 0.776 | 1.738 | 0.549 |
| Alcohol | Phytol | 6.18 | 0.162 | 0.195 | 0.242 | 0.531 | 0.352 | 0.335 | 0.291 | 0.189 |
| Docosanol | 10.93 | 0.129 | 0.068 | 0.122 | 0.13 | 0.073 | 0.128 | 0.137 | 0.247 | |
| Tetracosanol | 13.88 | 0.374 | 0.477 | 0.094 | 0.34 | 0.206 | 0.167 | 0.503 | 0.81 | |
| 9-C27-ol | 15.37 | 2.407 | 0.429 | 0.832 | 4.373 | 1.193 | 1.951 | 1.414 | 1.84 | |
| 1-Hexacosanol | 16.12 | ≤LOD | ≤LOD | ≤LOD | 0.001 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| 9-C28-ol + 10-C28-ol | 16.37 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | 0.037 | ≤LOD | ≤LOD | |
| 10-C29-ol | 17.27 | ≤LOD | 0.259 | ≤LOD | 0.107 | 0.014 | 0.24 | 0.128 | 0.062 | |
| 8,9-C27-diol | 18.56 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| 11-C31-ol | 19.42 | 0.007 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| 1-Triacontanol | 20.48 | 1.334 | 0.869 | 1.021 | 1.427 | 1.237 | 2.064 | 2.473 | 1.52 | |
| 1-Dotriacontanol | 23.49 | ≤LOD | ≤LOD | 0.184 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | |
| Subtotal | 4.413 | 2.297 | 2.495 | 6.909 | 3.075 | 4.922 | 4.946 | 4.668 | ||
| Aldehyde | Hexacosanal | 12.33 | 0.103 | 0.17 | 0.078 | 0.14 | 0.015 | 0.137 | 0.216 | 0.119 |
| Heptacosanal | 13.75 | 0.509 | 0.605 | 0.137 | 0.123 | 0.269 | 0.322 | 0.494 | 1.234 | |
| Octacosanal | 14.4 | 0.292 | 0.176 | 0.132 | 0.333 | 0.091 | 0.165 | 0.365 | 0.574 | |
| Triacontanal | 17.09 | 0.566 | 0.963 | 0.683 | 5.581 | 2.033 | 0.614 | 1.7 | 2.74 | |
| Dotriacontanal | 19.31 | 0.151 | 2.357 | 2.219 | 2.459 | 2.99 | 3.074 | 4.66 | 1.395 | |
| Subtotal | 1.621 | 4.271 | 3.249 | 8.636 | 5.398 | 4.312 | 7.435 | 6.062 | ||
| Carbohydrate | beta.-D-Allopyranose | 4.85 | 0.011 | ≤LOD | ≤LOD | 0.025 | 0.015 | 0.014 | 0.012 | 0.017 |
| Myo-Inositol | 5.8 | ≤LOD | ≤LOD | 0.006 | 0.021 | 0.023 | 0.01 | ≤LOD | ≤LOD | |
| Carbohydrate | 9.55 | 0.195 | 0.183 | 0.121 | 0.397 | 0.165 | ≤LOD | 0.13 | 0.174 | |
| Subtotal | 0.206 | 0.183 | 0.127 | 0.443 | 0.203 | 0.024 | 0.142 | 0.191 | ||
| Sesquiterpene | Abscisic acid | 6.61 | 0.048 | 0.075 | 0.045 | 0.056 | 0.082 | 0.067 | 0.037 | 0.069 |
| Ester | Octacosanoate | 19.17 | 0.75 | 3.068 | 1.062 | 1.518 | 0.993 | 3.26 | 5.249 | 1.015 |
| Ketone | Nonacosane-8,10-dione | 18.65 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD |
| Hentriacontane-10,12-dione | 21.27 | 2.567 | 2.918 | 1.334 | 3.441 | 2.297 | 0.583 | 3.858 | 6.001 | |
| Subtotal | 2.567 | 2.918 | 1.334 | 3.441 | 2.297 | 0.583 | 3.858 | 6.001 | ||
| Phenolic acid | Syringic acid | 4.3 | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD | ≤LOD |
| p-Coumaric acid | 4.65 | 0.031 | 0.004 | 0.024 | 0.042 | 0.034 | 0.011 | 0.021 | 0.02 | |
| Methyl caffeate | 5.11 | 0.055 | 0.107 | 0.025 | 0.086 | 0.029 | 0.068 | 0.083 | 0.009 | |
| Ferulic acid | 5.65 | ≤LOD | 0.002 | ≤LOD | ≤LOD | 0.015 | ≤LOD | ≤LOD | ≤LOD | |
| Subtotal | 0.086 | 0.113 | 0.049 | 0.128 | 0.078 | 0.079 | 0.104 | 0.029 | ||
| Tocopherols | alpha.-Tocopherol | 18.32 | 0.137 | 0.218 | 0.154 | 0.192 | 0.234 | 0.284 | 0.2 | ≤LOD |
| Total | 41.553 | 65.611 | 38.913 | 55.74 | 45.25 | 50.148 | 49.053 | 45.619 |
References
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. Biol. Plant Cuticle 2008, 23, 145–181. [Google Scholar]
- Kolb, C.A.; Kopecký, J.; Riederer, M.; Pfündel, E.E. UV screening by phenolics in berries of grapevine (Vitis vinifera). Funct. Plant Biol. 2003, 30, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Kothari, S.L.; Rathore, M.; Gour, V. Properties, variations, roles, and potential applications of epicuticular wax: A review. Turkish J. Bot. 2018, 42, 135–149. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Klavins, M. Surface wax composition of wild and cultivated Northern berries. Agron. Res. 2019, 17, 1337–1345. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Tech. 2014, 87, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Pensec, F.; Paczkowski, C.; Grabarczyk, M.; Woźniak, A.; Bénard-Gellon, M.; Bertsch, C.; Chong, J.; Szakiel, A. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. J. Agric. Food Chem. 2014, 62, 7998–8007. [Google Scholar] [CrossRef]
- Weingärtner, O.; Baber, R.; Teupser, D. Plant sterols in food: No consensus in guidelines. Biochem. Biophys. Res. Commun. 2014, 446, 811–813. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Yadav, J.; Datta, M.; Gour, V.S. Developing hydrophobic paper as a packaging material using epicuticular wax: A sustainable approach. Biol. Res. 2014, 9, 5066–5072. [Google Scholar] [CrossRef] [Green Version]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Klavins, L.; Klavina, L.; Huna, A.; Klavins, M. Polyphenols, carbohydrates and lipids in berries of Vaccinium species. Environ. Exp. Biol. 2015, 13, 147–158. [Google Scholar]
- Kovats, E. Gas chromatographic characterization of organic compounds. I. Retention indexes of aliphatic halides, alcohols, aldehydes, and ketones. Helv. Chim. Acta 1958, 41, 1915. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffree, C.E. The fine structure of the plant cuticle. Biol. Plant Cuticle 2006, 23, 11–25, 182–215. [Google Scholar]
- Trivedi, P.; Karppinen, K.; Klavins, L.; Kviesis, J.; Sundqvist, P.; Nguyen, N.; Heinonen, E.; Klavins, M.; Jaakola, L.; Väänänen, J.; et al. Compositional and morphological analyses of wax in northern wild berry species. Food Chem. 2019, 15, 295, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Hart, H.T.; Wollenweber, E. The systematic and evolutionary significance of exudate flavonoids in Aeonium. Phytochemistry 1995, 39, 805–813. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Cao, S.; Fang, X.; Chen, H.; Xiao, S. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef]
- Ahmed, A.S.; McGaw, L.J.; Moodley, N.; Naidoo, V.; Eloff, J.N. Cytotoxic, antimicrobial, antioxidant, antilipoxygenase activities and phenolic composition of Ozoroa and Searsia species (Anacardiaceae) used in South African traditional medicine for treating diarrhoea. S. Afr. J. Bot. 2014, 95, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.C.; Morais-Braga, M.F.; Guedes, G.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.; Coutinho, H.D. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed. Pharmacother. 2014, 68, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Seong, E.S.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Evaluation of phenolic compounds and antimicrobial activities in transgenic Codonopsis lanceolata plants via overexpression of the γ-tocopherol methyltransferase (γ-tmt) gene. S. Afr. J. Bot. 2017, 109, 25–33. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. A focus on the biosynthesis and composition of cuticle in fruits. J. Agric. Food Chem. 2015, 63, 4005–4019. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Bi, Y.; Chen, S.; Li, Y.; Wang, Y.; Ge, Y.; Ding, B.; Li, Y.; Zhang, Z. Chemical composition and antifungal activity of cuticular wax isolated from Asian pear fruit (cv. Pingguoli). Sci. Hortic. 2011, 129, 577–582. [Google Scholar] [CrossRef]
- Leliaert, F.; Verbruggen, H.; Zechman, F.W. Into the deep: New discoveries at the base of the green plant phylogeny. BioEssays 2011, 33, 683–692. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Rogers, L.M.; Li, D.; Hwang, C.S.; Flaishman, M.A. Surface signalling in pathogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 4080–4087. [Google Scholar] [CrossRef] [Green Version]
- Podila, G.K.; Rogers, L.M.; Kolattukudy, P.E. Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 1993, 103, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lü, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiol. Plant 2012, 146, 15–25. [Google Scholar] [CrossRef]
- Guo, Y.; He, Y.; Guo, N.; Gao, J.; Ni, Y. Variations of the composition of the leaf cuticular wax among Chinese populations of Plantago major. Chem. Biodivers. 2015, 12, 627–636. [Google Scholar] [CrossRef]
- Hedge, Y.; Kolattukudy, P.E. Cuticular waxes relieve self inhibition of germination and appressorium formation by the conidia of Manaporthe grisea. Physiol. Mol. Plant Pathol. 1997, 51, 75–84. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Vogg, G.; Riederer, M. The positional sterile (ps) mutation affects cuticular transpiration and wax biosynthesis of tomato fruits. J. Plant Physiol. 2011, 168, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Rao, J.; Huber, D.J.; Chang, X.; Xin, F. Wax composition of ‘Red Fuji’ apple fruit during development and during storage after 1-methylcyclopropene treatment. Hortic. Environ. Biotechnol. 2012, 53, 288–297. [Google Scholar] [CrossRef]
- Liu, D.C.; Zeng, Q.; Ji, Q.X.; Liu, C.F.; Liu, S.B.; Liu, Y. A comparison of the ultrastructure and composition of fruits’ cuticular wax from the wild-type ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck cv. Newhall) and its glossy mutant. Plant Cell Rep. 2012, 31, 2239–2246. [Google Scholar] [CrossRef]
- Dobson, G.; Shrestha, M.; Hilz, H.; Karjalainen, R.; McDougall, G.; Stewart, D. Lipophilic components in black currant seed and pomace extracts. Eur. J. Lipid Sci. Technol. 2012, 114, 575–582. [Google Scholar] [CrossRef]



| Studied berry | Family | Genus | Variety | Wax, mg Berry−1 |
|---|---|---|---|---|
| Hawthorn | Rosaceae | Crataegus | 1.43 ab ± 0.09 | |
| Rowanberry | Rosaceae | Sorbus | 1.48 ab ± 0.09 | |
| Gaultheria | Ericaceae | Gaultheria | 0.65 c ± 0.02 | |
| Black crowberry | Ericaceae | Empetrum | 1.71 a ± 0.11 | |
| Bog bilberry | Ericaceae | Vaccinium | 0.95 b ± 0.09 | |
| Bilberry | Ericaceae | Vaccinium | 0.63 c ± 0.05 | |
| Lingonberry | Ericaceae | Vaccinium | 1.89 a ± 0.09 | |
| American cranberry | Ericaceae | Vaccinium | 1.46 ab ± 0.12 | |
| Blueberry | Ericaceae | Vaccinium | ‘Blue crop’ | 0.74 b ± 0.04 |
| ‘Blue gold’ | 0.67 c ± 0.03 | |||
| ‘Chandler’ | 0.83 b ± 0.05 | |||
| ‘Chippewa’ | 0.90 b ± 0.07 | |||
| ‘Duke’ | 0.57 c ± 0.02 | |||
| ‘North blue’ | 0.65 c ± 0.02 | |||
| ‘Patriot’ | 0.84 b ± 0.03 | |||
| ‘Polaris’ | 0.87 b ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klavins, L.; Klavins, M. Cuticular Wax Composition of Wild and Cultivated Northern Berries. Foods 2020, 9, 587. https://doi.org/10.3390/foods9050587
Klavins L, Klavins M. Cuticular Wax Composition of Wild and Cultivated Northern Berries. Foods. 2020; 9(5):587. https://doi.org/10.3390/foods9050587
Chicago/Turabian StyleKlavins, Linards, and Maris Klavins. 2020. "Cuticular Wax Composition of Wild and Cultivated Northern Berries" Foods 9, no. 5: 587. https://doi.org/10.3390/foods9050587