Study of the Effects Induced by Ball Milling Treatment on Different Types of Hydrocolloids in a Corn Starch–Rice Flour System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
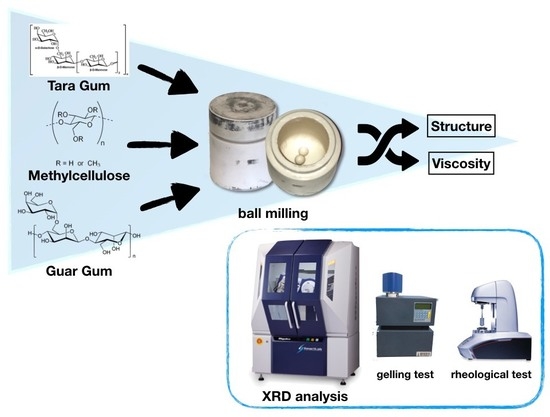
2.2. Ball Milling Treatment
2.3. X-ray Diffraction Experiments
2.4. Fourier Transform Infrared Analysis
2.5. Gelling Capacity of Hydrocolloids
2.6. Starch/Flour–Hydrocolloid Blend Preparation
2.7. Pasting Properties of Starch/Flour–Hydrocolloid Blends
2.8. Viscoelastic Properties of Starch/Flour–Hydrocolloid Blends
2.9. Statistical Analysis
3. Results and Discussion
3.1. Structural and Viscosity Properties of the Single Hydrocolloids
3.2. Properties of Binary Hydrocolloid–Starch Blends
3.3. Pasting Properties of Ternary Hydrocolloid–Hydrocolloid–Starch Blends
3.4. Viscoelastic Properties of Hydrocolloids Starch Gels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biliaderis, C.G.; Arvanitoyannis, I.; Izydorczyk, M.S.; Prokopowich, D.J. Effect of hydrocolloids on gelatinization and structure formation in concentrated waxy maize and wheat starch gels. Starch/Staerke 1997, 49, 278–283. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Rosell, C.M.; Yokoyama, W.; Shoemaker, C. Rheology of different hydrocolloids-rice starch blends. Effect of successive heating-cooling cycles. Carbohydr. Polym. 2011, 84, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Fang, Y.; Cao, Y.; Liao, H.; Nishinari, K.; Phillips, G.O. Hydrocolloid-food component interactions. Food Hydrocoll. 2017, 68, 149–156. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J.; Fauconnier, M.L. Influence of different hydrocolloids on dough thermo-mechanical properties and in vitro starch digestibility of gluten-free steamed bread based on potato flour. Food Chem. 2018, 239, 1064–1074. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and nutritional challenges, and novelty in gluten-free breadmaking: A review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J.; Naikwadi, N.N.; Variya, B.C. Galactomannan: A versatile biodegradable seed polysaccharide. Int. J. Biol. Macromol. 2013, 60, 83–92. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, J.Y.; Choi, J.; Kim, Y.C.; Shin, M. Pasting properties of non-waxy rice starch–hydrocolloid mixtures. Starch/Staerke 2006, 58, 223–230. [Google Scholar] [CrossRef]
- Rojas, J.A.; Rosell, C.M.; Benedito De Barber, C. Pasting properties of different wheat flour-hydrocolloid systems. Food Hydrocoll. 1999, 13, 27–33. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Macnaughtan, W.; Foster, T.J. The effect of ball milling and rehydration on powdered mixtures of hydrocolloids. Carbohydr. Polym. 2014, 102, 978–985. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Li, Y.; Huang, Q. Fabrication of milled cellulose particles-stabilized Pickering emulsions. Food Hydrocoll. 2018, 77, 427–435. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, K.; Zhou, M.; Wei, Y.J.; Cheng, F.; Lin, Y.; Zhu, P.X. High-Performance Starch Films Reinforced With Microcrystalline Cellulose Made From Eucalyptus Pulp via Ball Milling and Mercerization. Starch/Staerke 2019, 71, 1800218. [Google Scholar] [CrossRef]
- Ramadhan, K.; Foster, T.J. Effects of ball milling on the structural, thermal, and rheological properties of oat bran protein flour. J. Food Eng. 2018, 229, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Martínez, M.M.; Macias, A.K.; Belorio, M.L.; Gómez, M. Influence of marine hydrocolloids on extruded and native wheat flour pastes and gels. Food Hydrocoll. 2015, 43, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Liu, M.; Chen, J.; Han, F.; Gao, C.; Zhang, B. Synthesis and characterization of carboxymethyl guar gum and rheological properties of its solutions. Carbohydr. Polym. 2012, 88, 1015–1022. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Mariani, A.; Nuvoli, L.; Sanna, D.; Alzari, V.; Nuvoli, D.; Rassu, M.; Malucelli, G. Semi-interpenetrating polymer networks based on crosslinked poly (N-isopropyl acrylamide) and methylcellulose prepared by frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 437–443. [Google Scholar] [CrossRef]
- Hirrien, M.; Chevillard, C.; Desbrières, J.; Axelos, M.A.V.; Rinaudo, M. Thermogelation of methylcelluloses: New evidence for understanding the gelation mechanism. Polymer (Guildf) 1998, 39, 6251–6259. [Google Scholar] [CrossRef]
- Alloncle, M.; Doublier, J.L. Viscoelastic properties of maize starch/hydrocolloid pastes and gels. Top. Catal. 1991, 5, 455–467. [Google Scholar] [CrossRef]
- Bemiller, J.N. Pasting, paste, and gel properties of starch–hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch. Food Hydrocoll. 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Chaisawang, M.; Suphantharika, M. Effects of guar gum and xanthan gum additions on physical and rheological properties of cationic tapioca starch. Carbohydr. Polym. 2005, 61, 288–295. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2a. Functions of guar gums with different molecular weights on the gelatinization behavior of corn starch. Food Hydrocoll. 2005, 19, 15–24. [Google Scholar] [CrossRef]
- Collar, C.; Conte, P.; Fadda, C.; Piga, A. Gluten-free dough-making of specialty breads: Significance of blended starches, flours and additives on dough behaviour. Food Sci. Technol. Int. 2015, 21, 523–536. [Google Scholar] [CrossRef] [Green Version]
- Funami, T. Functions of Food Polysaccharides to Control the Gelatinization and Retrogradation Behaviors of Starch in an Aqueous System in Relation to the Macromolecular Characteristics of Food Polysaccharides. Food Sci. Technol. Res. 2010, 15, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Kohyama, K.; NIshinari, K. Cellulose Derivatives Effects on Gelatinization and Retrogradation of Sweet Potato Starch. J. Food Sci. 1992, 57, 128–131. [Google Scholar] [CrossRef]
- Hoefler, A.C. Hydrocolloids; Eagan Press Handbook Series, St.: Paul, MN, USA, 2004. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Jo, W.; Yoo, B. Rheological and microstructural characteristics of rice starch–tara gum mixtures. Int. J. Food Prop. 2017, 20, 1879–1889. [Google Scholar] [CrossRef]




| Samples | Corn Starch (g) | Rice Flour (g) | Guar Gum (g) | Tara Gum (g) | Methylcellulose (g) | Water (mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| UN | M | UN | M | UN | M | ||||
| Corn starch–rice flour | 1.75 | 1.75 | - | - | - | - | - | - | 25 |
| Binary mixtures | |||||||||
| starch–flour + UN-GG | 1.75 | 1.75 | 0.25 | - | - | - | - | - | 25 |
| starch–flour +M-GG | 1.75 | 1.75 | - | 0.25 | - | - | - | - | 25 |
| starch–flour + UN-TG | 1.75 | 1.75 | - | - | 0.25 | - | - | - | 25 |
| starch–flour + M-TG | 1.75 | 1.75 | - | - | - | 0.25 | - | - | 25 |
| starch–flour + UN-MC | 1.75 | 1.75 | - | - | - | - | 0.25 | - | 25 |
| starch–flour + M-MC | 1.75 | 1.75 | - | - | - | - | - | 0.25 | 25 |
| Ternary mixtures | |||||||||
| starch–flour + UN-MC + UN-GG | 1.75 | 1.75 | 0.125 | - | - | - | 0.125 | - | 25 |
| starch–flour + UN-MC + M-GG | 1.75 | 1.75 | - | 0.125 | - | - | 0.125 | - | 25 |
| starch–flour + M-MC + M-GG | 1.75 | 1.75 | - | 0.125 | - | - | - | 0.125 | 25 |
| starch–flour + UN-MC + UN-TG | 1.75 | 1.75 | - | - | 0.125 | - | 0.125 | - | 25 |
| starch–flour + UN-MC + M-TG | 1.75 | 1.75 | - | - | - | 0.125 | 0.125 | - | 25 |
| starch–flour + M-MC + M-TG | 1.75 | 1.75 | - | - | - | 0.125 | - | 0.125 | 25 |
| Samples | Parameters | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Viscosity (mPa⋅s) | Breakdown (mPa⋅s) | Final Viscosity (mPa⋅s) | Setback (mPa⋅s) | Peak Time min | Pasting Temperature °C | |||||||||||||||||||
| Corn starch–rice flour | 4917 | ± | 192 | a | 1300 | ± | 54 | a | 6360 | ± | 328 | a | 2743 | ± | 82 | a | 10.53 | ± | 0.0 | ef | 77.08 | ± | 0.60 | ns |
| Single Hydrocolloid + Starch–flour | ||||||||||||||||||||||||
| UN-GG | 10431 | ± | 430 | ef | 7168 | ± | 130 | cde | 9026 | ± | 86 | b | 5763 | ± | 385 | bc | 10.70 | ± | 0.0 | f | 78.23 | ± | 2.30 | ns |
| M-GG | 8681 | ± | 730 | c | 6337 | ± | 1080 | c | 9529 | ± | 645 | bc | 7185 | ± | 296 | cd | 10.57 | ± | 0.0 | f | 76.30 | ± | 2.90 | ns |
| UN-TG | 6732 | ± | 730 | b | 3854 | ± | 203 | b | 11,493 | ± | 1520 | c | 8615 | ± | 1201 | d | 10.60 | ± | 0.0 | f | 76.68 | ± | 0.00 | ns |
| M-TG | 6436 | ± | 613 | b | 3508 | ± | 185 | b | 11,651 | ± | 1641 | c | 8723 | ± | 1213 | d | 10.70 | ± | 0.0 | f | 76.60 | ± | 1.00 | ns |
| UN-MC | 9031 | ± | 716 | cd | 4224 | ± | 938 | b | 7644 | ± | 5 | ab | 2837 | ± | 216 | a | 10.00 | ± | 0.2 | abc | 76.33 | ± | 0.60 | ns |
| M-MC | 9115 | ± | 270 | cd | 4362 | ± | 412 | b | 7912 | ± | 186 | ab | 3158 | ± | 328 | a | 10.07 | ± | 0.1 | c | 76.65 | ± | 1.20 | ns |
| Pairs of Hydrocolloids + Starch–flour | ||||||||||||||||||||||||
| UN-MC + UN-GG | 10,428 | ± | 107 | ef | 6586 | ± | 35 | cd | 7299 | ± | 370 | ab | 3457 | ± | 228 | a | 10.33 | ± | 0.0 | de | 78.20 | ± | 1.20 | ns |
| UN-MC + M-GG | 13,336 | ± | 434 | g | 10,527 | ± | 533 | g | 7277 | ± | 231 | ab | 4468 | ± | 132 | ab | 10.04 | ± | 0.0 | bc | 78.58 | ± | 0.60 | ns |
| M-MC + M-GG | 12,980 | ± | 129 | g | 10,934 | ± | 18 | g | 6480 | ± | 2948 | a | 4434 | ± | 2801 | ab | 9.80 | ± | 0.2 | a | 78.60 | ± | 0.50 | ns |
| UN-MC + UN-TG | 9778 | ± | 454 | de | 7526 | ± | 260 | de | 8246 | ± | 204 | ab | 5994 | ± | 9 | bc | 10.17 | ± | 0.0 | cd | 78.70 | ± | 1.80 | ns |
| UN-MC + M-TG | 10,573 | ± | 134 | ef | 7981 | ± | 362 | e | 8619 | ± | 88 | ab | 6028 | ± | 408 | bc | 10.10 | ± | 0.0 | c | 79.45 | ± | 0.60 | ns |
| M-MC + M-TG | 10,948 | ± | 202 | f | 9235 | ± | 418 | f | 7445 | ± | 148 | ab | 5731 | ± | 68 | bc | 9.84 | ± | 0.2 | ab | 77.83 | ± | 1.60 | ns |
| Samples | Storage Modulus G′ (Pa) | Loss Modulus G″ (Pa) | Loss Tangent tan δ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn starch–rice flour | 1449 | ± | 191 | abc | 147 | ± | 0 | a | 0.103 | ± | 0.013 | ns |
| Single Hydrocolloid + Starch–flour | ||||||||||||
| UN-GG | 2024 | ± | 320 | d | 234 | ± | 12 | ab | 0.117 | ± | 0.012 | ns |
| M-GG | 1222 | ± | 227 | a | 151 | ± | 31 | a | 0.124 | ± | 0.002 | ns |
| UN-TG | 1785 | ± | 105 | cd | 315 | ± | 66 | ab | 0.176 | ± | 0.026 | ns |
| M-TG | 2887 | ± | 173 | e | 635 | ± | 335 | c | 0.217 | ± | 0.103 | ns |
| UN-MC | 1254 | ± | 161 | ab | 212 | ± | 20 | ab | 0.170 | ± | 0.005 | ns |
| M-MC | 1287 | ± | 230 | ab | 239 | ± | 66 | ab | 0.185 | ± | 0.018 | ns |
| Pairs of Hydrocolloids + Starch–flour | ||||||||||||
| UN-MC + UN-GG | 1594 | ± | 101 | abcd | 245 | ± | 50 | ab | 0.153 | ± | 0.021 | ns |
| UN-MC + M-GG | 1705 | ± | 201 | bcd | 252 | ± | 3 | ab | 0.149 | ± | 0.016 | ns |
| M-MC + M-GG | 1816 | ± | 34 | cd | 284 | ± | 37 | ab | 0.157 | ± | 0.018 | ns |
| UN-MC + UN-TG | 1379 | ± | 14 | abc | 237 | ± | 6 | ab | 0.172 | ± | 0.002 | ns |
| UN-MC + M-TG | 1751 | ± | 523 | cd | 312 | ± | 113 | ab | 0.177 | ± | 0.012 | ns |
| M-MC + M-TG | 1942 | ± | 26 | d | 399 | ± | 110 | b | 0.206 | ± | 0.059 | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuvoli, L.; Conte, P.; Garroni, S.; Farina, V.; Piga, A.; Fadda, C. Study of the Effects Induced by Ball Milling Treatment on Different Types of Hydrocolloids in a Corn Starch–Rice Flour System. Foods 2020, 9, 517. https://doi.org/10.3390/foods9040517
Nuvoli L, Conte P, Garroni S, Farina V, Piga A, Fadda C. Study of the Effects Induced by Ball Milling Treatment on Different Types of Hydrocolloids in a Corn Starch–Rice Flour System. Foods. 2020; 9(4):517. https://doi.org/10.3390/foods9040517
Chicago/Turabian StyleNuvoli, Luca, Paola Conte, Sebastiano Garroni, Valeria Farina, Antonio Piga, and Costantino Fadda. 2020. "Study of the Effects Induced by Ball Milling Treatment on Different Types of Hydrocolloids in a Corn Starch–Rice Flour System" Foods 9, no. 4: 517. https://doi.org/10.3390/foods9040517