Non-Thermal Ultrasonic Extraction of Polyphenolic Compounds from Red Wine Lees
Abstract
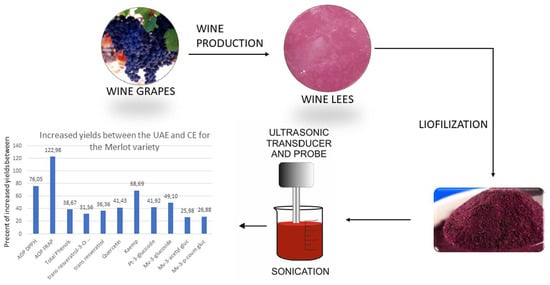
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Preparation of Wine Lees for Extraction
2.4. Extraction of Bioactive Compounds from Wine Lees
2.4.1. Conventional Extraction
2.4.2. High-Intensity Ultrasound-Assisted Extraction (UAE)
2.5. Bioactive Potential of Extracted Compounds
2.5.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.2. 2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.6. Determination of Total Phenolic Content
2.7. HPLC-DAD-ESI-MS/MS Analyses for Phenolic Characterization
2.8. Experimental Design and Statistical Analysis
t × Ds + a9 × t × Cw + a10 × Ds × Cw + a11 × A2 + a12 × t2 + a13 × A × t × Ds + a14 × A × t ×
Cw + a15×A+Ds×Cw + a16×t × Ds × Cw + a17 × A2 × Ds + a18 × A2 × Cw + a19 × t2 × Ds + a20
× t2 × Cw + a21 × A × t × Ds × Cw + a22 × A2 × Ds × Cw + a23 × t2 × Ds × Cw
3. Results
3.1. Antioxidant Potential and Total Phenolic Content Extracts
(p < 0.0001, R2 = 0.9437)
A × Ds − 1.26 × t × Ds − 1.48 × Ds × Cw + 0.47 × A2 −3.77 × t2 + 1.85 × A2 × D
(p < 0.0001, R2 = 0.9476)
Ds + 0.123 × A × Cw × D + 1.68 × t × Ds + 2.98 × t × Cw − 1.68 × Ds × Cw − 2.00 × A2
− 1.06 × A × Ds × Cw − 0.26 × A2 × Ds −2.77 × A2 × Cw + 1.68 × A2 × Ds × Cw
(p < 0.0001, R2 = 0.6963)
3.2. Individual Phenolics of CW and UAE Extracts
3.2.1. Trans-Resveratrol Glucoside
0.01 × A × CW − 0.01 × t × Ds − 0.01 × t × Cw − 0.00 × Ds × Cw −0.00 × A2 + 0.00 × t2
+ 0.00 × A × Ds × Cw + 0.01 × t × Ds × Cw − 0.01 × A2 × Ds + 0.00 × A2 × Cw − 0.00 ×
t2 × Ds − 0.00 × t2 × Cw + 0.01 × A2 × Ds × Cw + 0.00 × t2 × Ds × Cw
(p < 0.0001, R2 = 0.9997)
Cw − 0.00 × t × Ds + 0.00 × t × Cw − 0.00 × Ds × Cw + 0.00 × A2 + 0.00 × t2 − 0.00 × A
× Ds × Cw + 0.00 × A2 × Ds + 0.00 × A2 × Cw + 0.00 × t2 × Ds + 0.00 × t2 × Cw + 0.00 × A2 × Ds ×Cw
(p < 0.0001, R2 = 0.9844)
3.2.2. Quercetin and Kaempferol
A × Cw −0.03 × t × Ds + 0.01 × t × Cw − 0.03 × Ds × Cw − 0.01 × A2 + 0.00 × t2 + 0.01
× A × Ds × Cw + 0.01 × t × Ds × Cw − 0.02 × A2 × Ds + 0.03 × A2 × Cw + 0.01 × t2
× Ds+ 0.00 × t2 × Cw + 0.04 × A2 × Ds × Cw + 0.02 × t2 × Ds × Cw
(p < 0.0001, R2 = 0.9241)
× t × Cw −0.00 × Ds × Cw + 0.00 × t × Ds × Cw
(p < 0.0001, R2 = 0.9797)
3.2.3. Petunidin-3-O-Glucoside
(p < 0.0001, R2 = 0.9676)
3.2.4. Malvidin-3-Glucoside
A×Ds + 0.04 × A × Cw − 0.08 × t × Ds + 0.03 × t × Cw − 0.14 × Ds × Cw + 0.08 × A2 +
0.05 × t2 + 0.01 × A × Ds × Cw + 0.10 × A2 × Ds + 0.10 × A2 × Cw + 0.10 × t2 × Ds +
0.09 × t2 × Cw + 0.10 × A2 × Ds × Cw
(p < 0.0001, R2 = 0.8379)
− 0.07 × A×Ds + 0.05 × A × Cw − 0.04 × t × Ds + 0.01 × t × Cw − 0.04 × Ds × Cw − 0.03
× A2 + 0.04 × t2 + 0.04 × A × Ds × Cw + 0.00 × t × Ds × Cw − 0.03 × A2 × Ds + 0.13 × A2
× Cw + 0.07 × t2 × Ds+ 0.01 × t2 × Cw + 0.10 × A2 × Ds × Cw − 0.03 × t2 × Ds × Cw
(p < 0.0001, R2 = 0.7747)
0.29 × Cw − 0.01 × A × t −0.03 × A × Ds − 0.01 × A × Cw − 0.03 × t × Ds + 0.00 × t ×
Cw − 0.01 × Ds × Cw + 0.02 × A2 + 0.03 × A × t × Cw b2
(p < 0.0001, R2 = 0.9750)
3.3. Optimization of UAE Extraction Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 1993; p. 362. [Google Scholar]
- Jurčević, L.I.; Dora, M.; Guberović, M.; Petras, S.; Brnčić, S.R.; Đikić, D. Polyphenols from wine lees as a novel functional bioactive compound in the protection against oxidative stress and hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar]
- Council Regulation (EC) No 479/2008, on the common organisation of the market in wine, amending Regulations (EC) No 1493/1999, (EC) No 1782/2003, (EC) No 1290/2005, (EC) No 3/2008 and repealing Regulations (EEC) No 2392/86 and (EC) No 1493/1999. Off. J. Eur. Union 2008, 51, 1–62.
- Cheynier, V.; Basire, N.; Rigaud, J. Mechanism of trans-caffeoyltartaric acid and catechin oxidation in model solutions containing grape polyphenoloxidase. J. Agric. Food Chem. 1989, 37, 1069–1071. [Google Scholar] [CrossRef]
- Da Silva, J.M.R.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Slinkard, K.W.; Singleton, V. Phenol content of grape skins and the loss of ability to make anthocyanins by mutation. Vitis 1984, 23, 175–178. [Google Scholar]
- Souquet, J.M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Int. 2000, 33, 469–474. [Google Scholar] [CrossRef]
- Luque-Rodriguez, J.M.; Luque de Castro, M.D.; Perez-Juan, P. Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemaking residues. Bioresour. Technol. 2007, 98, 2705–2713. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food Bioprocess Technol. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Radošević, K.; Srcek, V.G.; Bubalo, M.C.; Brncic, S.R.; Takacs, K.; Redovnikovic, I.R. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Doka, O.; Bicanic, D. Determination of total polyphenolic content in red wines by means of the combined He-Ne laser optothermal window and Folin–Ciocalteu colorimetry assay. Anal. Chem. 2002, 74, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Suraski, A.; Aviram, M. White wine with red wine-like properties: Increased extraction of grape skin polyphenols improves the antioxidant capacity of the derived white wine. J. Agric. Food Chem. 2001, 49, 3164–3168. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Tsang, E.; Karumanchiri, A.; Diamandis, E.P.; Soleas, G.; Ng, E. Method to assay the concentrations of phenolic constituents of biological interest in wines. Anal. Chem. 1996, 68, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Lamuela-Raventos, R.M.; de la Torre-Boronat, M.C.; Planas, J.M. Determination of trans-resveratrol in plasma by HPLC. Anal. Chem. 1999, 71, 747–750. [Google Scholar] [CrossRef]
- Paceasciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Dudley, J.I.; Lekli, I.; Mukherjee, S.; Das, M.; Bertelli, A.A.A.; Das, D.K. Does white wine qualify for French Paradox? Comparison of the cardioprotective effects of red and white wines and their constituents: Resveratrol, tyrosol, and hydroxytyrosol (Retracted article. See vol. 60, pg. 2767, 2012). J. Agric. Food Chem. 2008, 56, 9362–9373. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective actions of grape polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Xia, E.; He, X.; Li, H.; Wu, S.; Li, S.; Deng, G. Chapter 5—Biological activities of polyphenols from grapes. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 47–58. [Google Scholar]
- Stoclet, J.C.; Chataigneau, T.; Ndiaye, M.; Oak, M.H.; El Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, J.; Kern, M.; Pahlke, G.; Vatter, S.; Marko, D. Biological activities of malvidin, a red wine anthocyanidin. Mol. Nutr. Food Res. 2006, 50, 390–395. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Murali, A.; Shetty, P.H. Petunidin as a competitive inhibitor of acylated homoserine lactones in Klebsiella pneumoniae. RSC Adv. 2016, 6, 2592–2601. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Zhijing, Y.; Shavandi, A.; Harrison, R.; Bekhit, A.E.A. Characterization of phenolic compounds in wine lees. Antioxidants 2018, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, Z.; Dinya, Z. Examination of non-volatile organic compounds in red wines made in Eger. Microchem. J. 2000, 67, 57–62. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Jauregui, O.; Lamuela-Raventos, R.M.; Jaeger, W.; Miksits, M.; Covas, M.I.; Andres-Lacueva, C. Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal. Chem. 2005, 77, 3149–3155. [Google Scholar] [CrossRef]
- Feijóo, O.; Moreno, A.; Falqué, E. Content of trans- and cis-resveratrol in Galician white and red wines. J. Food Compos. Anal. 2008, 21, 608–613. [Google Scholar] [CrossRef]
- Stratil, P.; Kuban, V.; Fojtova, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2008, 26, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Sulfaro, V.; Trombetta, D.; Saija, A.; Tomaino, A. Radical-scavenging capacity of several Italian red wines. Food Chem. 2007, 103, 75–81. [Google Scholar] [CrossRef]
- De Beer, D.; Joubert, E.; Marais, J.; Manley, M. Unravelling the total antioxidant capacity of pinotage wines: Contribution of phenolic compounds. J. Agric. Food Chem. 2006, 54, 2897–2905. [Google Scholar] [CrossRef]
- Fiori, L.; de Faveri, D.; Casazza, A.A.; Perego, P. Grape by-products: Extraction of polyphenolic compounds using supercritical CO2 and liquid organic solvent. CYTA-J. Food 2009, 7, 163–171. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar]
- Novak, I.; Janeiro, P.; Seruga, M.; Oliveira-Brett, A.M. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Anal. Chim. Acta 2008, 630, 107–115. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Priego-Capote, F.; Luque de Castro, M.D. Simultaneous ultrasound-assisted emulsification−extraction of polar and nonpolar compounds from solid plant samples. Anal. Chem. 2007, 79, 6767–6774. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of maceration temperature in red wine vinification on extraction of phenolics from berry skins and seeds of grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodriguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Agostini, F.; Bertussi, R.A.; Agostini, G.; Atti Dos Santos, A.C.; Rossato, M.; Vanderlinde, R. Supercritical extraction from vinification residues: Fatty acids, alpha-tocopherol, and phenolic compounds in the oil seeds from different varieties of grape. Sci. World J. 2012, 2012, 790486. [Google Scholar] [CrossRef] [Green Version]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Effects of pulsed electric fields on the extraction of phenolic compounds during the fermentation of must of Tempranillo grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Brncic, M.; Tripalo, B.; Rimac Brncic, S.; Karlovic, S.; Zupan, A.; Herceg, Z. Evaluation of textural properties for whey enriched direct extruded and puffed corn based products. Bulg. J. Agric. Sci. 2009, 15, 204–213. [Google Scholar]
- Hegedušić, V.; Herceg, Z.; Rimac, S. Rheological properties of carboxymethylcellulose and whey model solutions before and after freezing. Food Technol. Biotechnol. 2000, 38, 19–26. [Google Scholar]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Rimac Brnčić, S.; Vitali Čepo, D. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT Food Sci. Technol. 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Galanakis, C.M.; Brnčić, M.; Grimi, N.; Boussetta, N.; Mota, M.J.; Saraiva, J.A.; Patras, A.; Tiwari, B.; Barba, F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015, 77, 743–752. [Google Scholar] [CrossRef]
- Dujmic, F.; Brncic, M.; Karlovic, S.; Bosiljkov, T.; Jezek, D.; Tripalo, B.; Mofardin, I. Ultrasound-assisted infrared drying of pear slices: Textural issues. J. Food Process Eng. 2013, 36, 397–406. [Google Scholar] [CrossRef]
- Mulet, A.; Cárcel, J.; Garcia-Perez, J.V.; Riera, E. Ultrasound-Assisted Hot Air Drying of Foods; Springer: New York, NY, USA, 2010; pp. 511–534. [Google Scholar]
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Carbonell Capella, J.; Šic Žlabur, J.; Rimac Brnčić, S.F.B.; Grimi, N.; Koubaa, M.; Brnčić, M.; Vorobiev, E. Electrotechnologies, microwaves, and ultrasounds combined with binary mixtures of ethanol and water to extract steviol glycosides and antioxidant compounds from Stevia rebaudiana leaves. J. Food Process. Preserv. 2017, 41, 5. [Google Scholar] [CrossRef]
- Herceg, Z.; Brnčić, M.; Režek Jambrak, A.; Rimac Brnčić, S.; Badanjak, M.; Sokolić, I. Possibility of application high intensity ultrasound in milk industry. Mljekarstvo 2009, 59, 65–69. [Google Scholar]
- Ćurko, N.; Ganić, K.K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Elez Garofulić, I.; Bosiljkov, T.; Ježek, D.; Brnčić, M. Comparison of conventional and ultrasound-assisted extraction techniques on mass fraction of phenolic compounds from sage (Salvia officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; de Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. Power ultrasound processing of cantaloupe melon juice: Effects on quality parameters. Food Res. Int. 2012, 48, 41–48. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Grassino, A.N.; Halambek, J.; Djaković, S.; Rimac Brnčić, S.; Dent, M.; Grabarić, Z. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Grassino, A.N.; Barba, F.J.; Brnčić, M.; Lorenzo, J.M.; Lucini, L.; Rimac Brnčić, S. Analytical tools used for the identification and quantification of pectin extracted from plant food matrices, wastes and by-products: A review. Food Chem. 2018, 266, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Multivariate optimisation of the microwave-assisted extraction of oleuropein and related biophenols from olive leaves. Anal. Bioanal. Chem. 2006, 385, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Serradilla, J.A.; Japón-Luján, R.; Luque de Castro, M.D. Simultaneous microwave-assisted solid–liquid extraction of polar and nonpolar compounds from alperujo. Anal. Chim. Acta 2007, 602, 82–88. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Sabotin, I.; Radoš, I.; Valentinčič, J.; Bosiljkov, T.; Brnčić, M.; Žnidaršič-Plazl, P. A comparative study of ultrasound, microwave and microreactor-assisted imidazolium-based ionic liquid synthesis. Green Process. Synth. 2013, 2, 579–590. [Google Scholar]
- Chemat, F.; Zille, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2013, 55, 570–594. [Google Scholar] [CrossRef]
- Cacciola, V.; Batllò, I.F.; Ferraretto, P.; Vincenzi, S.; Celotti, E. Study of the ultrasound effects on yeast lees lysis in winemaking. Eur. Food Res. Technol. 2012, 236, 311–317. [Google Scholar] [CrossRef]
- Garcia Martin, J.F.; Guillemet, L.; Feng, C.; Sun, D.W. Cell viability and proteins release during ultrasound-assisted yeast lysis of light lees in model wine. Food Chem. 2013, 141, 934–939. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of wine volatile phenols by yeast lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Vlyssides, A.G.; Israilides, C.J. Characterization of alcohol manufacturing industrial wastes in Greece. Fresenius Environ. Bull. 1997, 6, 699–704. [Google Scholar]
- Mercurio, M.D.; Smith, P.A. Tannin quantification in red grapes and wine: Comparison of polysaccharide- and protein-based tannin precipitation techniques and their ability to model wine astringency. J. Agric. Food Chem. 2008, 56, 5528–5537. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, R.P.; Bravo, M.N.; Pires, M.M.; Serra, A.T.; Duarte, C.M.; Boas, L.V.; Bronze, M.R. Phenolic content and antioxidant activity of moscatel dessert wines from the Setúbal region in portugal. Food Anal. Methods 2009, 2, 149–161. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Hermosín-Gutiérrez, I.; Boros, B.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Dörnyei, Á.; Kilár, F. Phenolic compounds and antioxidant activity of Macedonian red wines. J. Food Compos. Anal. 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Wine and Must Analysis. J. Chromatogr. Sci. 1974, 12, 19. [Google Scholar] [CrossRef]
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wines, 2nd ed.; Wiley-Interescience: New York, NY, USA, 1988. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bertalanic, L.; Kosmerl, T.; Ulrih, N.P.; Cigic, B. Influence of solvent composition on antioxidant potential of model polyphenols and red wines determined with 2,2-diphenyl-1-picrylhydrazyl. J. Agric. Food Chem. 2012, 60, 12282–12288. [Google Scholar] [CrossRef]
- Garcia-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of grape and wine anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 325–434. [Google Scholar]







| A | t | Ds | AOP DPPH | AOP FRAP | TPC | trans-resv-3-O-gluc | trans Resveratrol | Quercetin | Kaempferol | Pt-3-Glucoside | Mv-3-Glucoside | Mv-3-acetyl gluc | Mv-3-p-coum gluc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 38.79 | 322.10 | 22 | 56.43 ± 0.31 | 58.05 ± 1.21 | 56.52 ± 1.11 | 0.61 ± 0.00 | 0.04 ± 0.00 | 1.09 ± 0.02 | 0.13 ± 0.01 | 2.44 ± 0.05 | 2.46 ± 0.04 | 1.36 ± 0.01 | 0.79 ± 0.01 |
| 81.21 | 322.10 | 22 | 64.17 ± 0.22 | 68.76 ± 1.13 | 37.64 ± 1.52 | 0.68 ± 0.00 | 0.04 ± 0.00 | 1.23 ± 0.01 | 0.15 ± 0.02 | 3.00 ± 0.09 | 2.91 ± 0.01 | 1.64 ± 0.02 | 1.11 ± 0.02 |
| 38.79 | 1297.90 | 22 | 68.09 ± 0.11 | 76.99 ± 0.98 | 32.45 ± 1.21 | 0.69 ± 0.01 | 0.04 ± 0.01 | 1.25 ± 0.03 | 0.15 ± 0.01 | 3.09 ± 0.08 | 3.33 ± 0.02 | 1.57 ± 0.01 | 1.08 ± 0.03 |
| 81.21 | 1297.90 | 22 | 78.30 ± 0.61 | 92.74 ± 0.96 | 39.01 ± 1.09 | 0.78 ± 0.01 | 0.05 ± 0.01 | 1.37 ± 0.01 | 0.16 ± 0.03 | 3.41 ± 0.01 | 3.68 ± 0.03 | 1.74 ± 0.03 | 1.17 ± 0.01 |
| 30.00 | 810.00 | 22 | 60.98 ± 0.21 | 66.97 ± 0.99 | 54.30 ± 0.89 | 0.64 ± 0.00 | 0.04 ± 0.00 | 1.21 ± 0.02 | 0.15 ± 0.03 | 2.96 ± 0.11 | 3.22 ± 0.01 | 1.64 ± 0.01 | 1.00 ± 0.02 |
| 90.00 | 810.00 | 22 | 72.94 ± 0.11 | 82.29 ± 1.12 | 49.57 ± 0.75 | 0.72 ± 0.01 | 0.04 ± 0.01 | 1.31 ± 0.03 | 0.16 ± 0.01 | 3.22 ± 0.09 | 3.47 ± 0.04 | 1.64 ± 0.01 | 1.14 ± 0.01 |
| 60.00 | 120.00 | 22 | 48.39 ± 0.40 | 52.45 ± 1.50 | 50.42 ± 0.49 | 0.63 ± 0.00 | 0.04 ± 0.01 | 1.12 ± 0.01 | 0.13 ± 0.01 | 2.63 ± 0.08 | 2.99 ± 0.05 | 1.45 ± 0.01 | 1.03 ± 0.02 |
| 60.00 | 1500.00 | 22 | 76.98 ± 0.55 | 89.90 ± 1.11 | 43.74 ± 0.29 | 0.75 ± 0.00 | 0.05 ± 0.00 | 1.32 ± 0.02 | 0.17 ± 0.02 | 3.09 ± 0.11 | 3.41 ± 0.04 | 1.67 ± 0.20 | 1.12 ± 0.01 |
| 60.00 | 810.00 | 22 | 67.61 ± 0.51 | 77.31 ± 2.14 | 42.40 ± 1.93 | 0.67 ± 0.01 | 0.04 ± 0.00 | 1.21 ± 0.01 | 0.14 ± 0.01 | 3.01 ± 0.08 | 3.29 ± 0.04 | 1.68 ± 0.02 | 1.06 ± 0.01 |
| 38.79 | 322.10 | 40 | 48.64 ± 0.02 | 55.39 ± 2.10 | 49.13 ± 1.01 | 0.63 ± 0.01 | 0.04 ± 0.01 | 1.16 ± 0.01 | 0.15 ± 0.00 | 2.85 ± 0.03 | 2.97 ± 0.11 | 1.59 ± 0.11 | 1.02 ± 0.02 |
| 81.21 | 322.10 | 40 | 53.43 ± 0.05 | 58.32 ± 2.11 | 42.36 ± 0.99 | 0.68 ± 0.01 | 0.04 ± 0.02 | 1.10 ± 0.01 | 0.16 ± 0.01 | 3.07 ± 0.02 | 3.13 ± 0.09 | 1.69 ± 0.11 | 1.02 ± 0.02 |
| 38.79 | 1297.90 | 40 | 59.97 ± 0.20 | 66.48 ± 1.51 | 48.68 ± 0.84 | 0.64 ± 0.00 | 0.04 ± 0.01 | 1.09 ± 0.03 | 0.14 ± 0.01 | 2.87 ± 0.01 | 3.23 ± 0.09 | 1.61 ± 0.09 | 1.03 ± 0.01 |
| 81.21 | 1297.90 | 40 | 65.31 ± 0.31 | 82.36 ± 1.31 | 46.18 ± 0.85 | 0.68 ± 0.02 | 0.04 ± 0.00 | 1.03 ± 0.02 | 0.14 ± 0.02 | 2.41 ± 0.04 | 2.45 ± 0.05 | 1.46 ± 0.80 | 0.93 ± 0.01 |
| 30.00 | 810.00 | 40 | 55.93 ± 0.33 | 70.57 ± 1.89 | 46.49 ± 0.77 | 0.60 ± 0.01 | 0.03 ± 0.00 | 1.00 ± 0.02 | 0.13 ± 0.01 | 2.38 ± 0.02 | 2.64 ± 0.09 | 1.18 ± 0.11 | 0.94 ± 0.02 |
| 90.00 | 810.00 | 40 | 62.16 ± 0.11 | 71.25 ± 2.05 | 57.24 ± 0.22 | 0.66 ± 0.00 | 0.04 ± 0.01 | 0.85 ± 0.01 | 0.13 ± 0.03 | 2.90 ± 0.02 | 3.11 ± 0.11 | 0.32 ± 0.05 | 1.04 ± 0.01 |
| 60.00 | 120.00 | 40 | 38.93 ± 0.22 | 41.97 ± 1.13 | 51.92 ± 0.56 | 0.64 ± 0.01 | 0.05 ± 0.01 | 1.06 ± 0.03 | 0.14 ± 0.04 | 2.46 ± 0.01 | 2.74 ± 0.10 | 1.52 ± 0.09 | 0.94 ± 0.02 |
| 60.00 | 1500.00 | 40 | 63.99 ± 0.31 | 69.25 ± 2.98 | 48.31 ± 0.78 | 0.68 ± 0.01 | 0.04 ± 0.00 | 1.15 ± 0.02 | 0.15 ± 0.00 | 2.98 ± 0.02 | 3.15 ± 0.11 | 1.66 ± 0.08 | 0.99 ± 0.01 |
| 60.00 | 810.00 | 40 | 58.34 ± 0.62 | 66.39 ± 3.15 | 52.63 ± 1.08 | 0.67 ± 0.02 | 0.04 ± 0.00 | 1.16 ± 0.02 | 0.14 ± 0.00 | 2.92 ± 0.03 | 2.94 ± 0.16 | 1.52 ± 0.14 | 0.95 ± 0.09 |
| CE | 3600.00 | 44.21 ± 0.33 | 42.52 ± 1.13 | 32.95 ± 1.09 | 0.60 ± 0.01 | 0.030 ± 0.00 | 1.01 ± 0.02 | 0.10 ± 0.02 | 2.26 ± 0.03 | 2.39 ± 0.09 | 1.36 ± 0.09 | 0.93 ± 0.01 |
| A | t | Ds | AOP DPPH | AOP FRAP | TPC | trans-resv-3-O-gluc | trans Resveratrol | Quercetin | Kaempferol | Pt-3-Glucoside | Mv-3-Glucoside | Mv-3-acetyl gluc | Mv-3-p-coum gluc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 38.79 | 322.10 | 22 | 59.86 ± 0.11 | 68.83 ± 1.33 | 44.92 ± 0.78 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.17 ± 0.02 | 0.06 ± 0.01 | 1.27 ± 0.03 | 3.40 ± 0.05 | 1.30 ± 0.02 | 1.62 ± 0.02 |
| 81.21 | 322.10 | 22 | 67.20 ± 0.25 | 76.28 ± 1.25 | 41.34 ± 0.63 | 0.00 ± 0.00 | 0.12 ± 0.02 | 1.22 ± 0.03 | 0.06 ± 0.01 | 1.37 ± 0.02 | 3.70 ± 0.06 | 1.45 ± 0.01 | 1.64 ± 0.01 |
| 38.79 | 1297.90 | 22 | 70.07 ± 0.10 | 79.82 ± 1.29 | 40.48 ± 0.22 | 0.00 ± 0.00 | 0.11 ± 0.00 | 1.29 ± 0.02 | 0.05 ± 0.02 | 1.41 ± 0.03 | 3.78 ± 0.01 | 1.39 ± 0.01 | 1.66 ± 0.02 |
| 81.21 | 1297.90 | 22 | 79.76 ± 0.05 | 96.45 ± 1.34 | 43.70 ± 0.45 | 0.01 ± 0.00 | 0.13 ± 0.02 | 1.42 ± 0.04 | 0.08 ± 0.03 | 1.52 ± 0.01 | 4.24 ± 0.02 | 1.63 ± 0.02 | 1.80 ± 0.01 |
| 30.00 | 810.00 | 22 | 65.39 ± 0.09 | 73.34 ± 1.02 | 32.34 ± 0.36 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.22 ± 0.03 | 0.07 ± 0.01 | 1.30 ± 0.02 | 3.31 ± 0.03 | 1.36 ± 0.03 | 1.61 ± 0.02 |
| 90.00 | 810.00 | 22 | 73.58 ± 0.24 | 90.62 ± 1.27 | 32.61 ± 0.56 | 0.00 ± 0.00 | 0.11 ± 0.00 | 1.34 ± 0.02 | 0.06 ± 0.00 | 1.43 ± 0.04 | 3.96 ± 0.04 | 1.51 ± 0.02 | 1.74 ± 0.01 |
| 60.00 | 120.00 | 22 | 51.00 ± 0.11 | 56.14 ± 1.08 | 34.20 ± 0.68 | 0.00 ± 0.00 | 0.09 ± 0.01 | 1.11 ± 0.03 | 0.06 ± 0.01 | 1.15 ± 0.01 | 3.32 ± 0.05 | 1.18 ± 0.01 | 1.55 ± 0.02 |
| 60.00 | 1500.00 | 22 | 76.47 ± 0.22 | 96.75 ± 1.04 | 49.16 ± 0.71 | 0.00 ± 0.00 | 0.13 ± 0.02 | 1.35 ± 0.02 | 0.07 ± 0.01 | 1.41 ± 0.02 | 4.02 ± 0.01 | 1.54 ± 0.00 | 1.74 ± 0.00 |
| 60.00 | 810.00 | 22 | 71.68 ± 0.32 | 85.93 ± 1.44 | 48.09 ± 0.84 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.28 ± 0.03 | 0.06 ± 0.00 | 1.35 ± 0.02 | 3.75 ± 0.07 | 1.37 ± 0.02 | 1.66 ± 0.02 |
| 38.79 | 322.10 | 40 | 52.73 ± 0.22 | 55.63 ± 2.10 | 43.35 ± 1.21 | 0.00 ± 0.00 | 0.10 ± 0.00 | 1.14 ± 0.03 | 0.05 ± 0.01 | 1.16 ± 0.01 | 3.11 ± 0.02 | 1.24 ± 0.05 | 1.51 ± 0.02 |
| 81.21 | 322.10 | 40 | 58.77 ± 0.66 | 66.56 ± 1.57 | 37.09 ± 1.51 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.18 ± 0.01 | 0.07 ± 0.02 | 1.22 ± 0.02 | 3.26 ± 0.02 | 1.31 ± 0.04 | 1.55 ± 0.01 |
| 38.79 | 1297.90 | 40 | 62.74 ± 0.22 | 70.80 ± 1.45 | 43.53 ± 1.32 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.18 ± 0.02 | 0.06 ± 0.01 | 1.29 ± 0.01 | 3.37 ± 0.05 | 1.39 ± 0.04 | 1.57 ± 0.02 |
| 81.21 | 1297.90 | 40 | 69.40 ± 0.45 | 81.12 ± 2.01 | 54.06 ± 1.52 | 0.00 ± 0.00 | 0.12 ± 0.00 | 1.24 ± 0.04 | 0.07 ± 0.01 | 1.41 ± 0.02 | 3.71 ± 0.01 | 1.43 ± 0.03 | 1.61 ± 0.02 |
| 30.00 | 810.00 | 40 | 57.98 ± 0.25 | 64.99 ± 1.55 | 47.34 ± 1.03 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.18 ± 0.03 | 0.05 ± 0.02 | 1.27 ± 0.03 | 3.34 ± 0.02 | 1.37 ± 0.04 | 1.57 ± 0.01 |
| 90.00 | 810.00 | 40 | 64.14 ± 0.29 | 71.33 ± 1.36 | 37.84 ± 1.05 | 0.00 ± 0.00 | 0.11 ± 0.01 | 1.19 ± 0.02 | 0.06 ± 0.01 | 1.35 ± 0.01 | 3.63 ± 0.03 | 1.38 ± 0.03 | 1.59 ± 0.01 |
| 60.00 | 120.00 | 40 | 40.74 ± 0.35 | 44.24 ± 1.32 | 39.66 ± 1.10 | 0.00 ± 0.00 | 0.10 ± 0.00 | 1.08 ± 0.01 | 0.05 ± 0.00 | 1.10 ± 0.01 | 2.98 ± 0.00 | 1.21 ± 0.05 | 1.56 ± 0.01 |
| 60.00 | 1500.00 | 40 | 65.89 ± 0.38 | 73.05 ± 1.87 | 57.43 ± 1.21 | 0.00 ± 0.00 | 0.11 ± 0.00 | 1.24 ± 0.04 | 0.06 ± 0.00 | 1.34 ± 0.02 | 3.57 ± 0.04 | 1.19 ± 0.01 | 1.56 ± 0.02 |
| 60.00 | 810.00 | 40 | 59.95 ± 0.88 | 67.26 ± 2.18 | 48.86 ± 1.71 | 0.00 ± 0.00 | 0.09 ± 0.00 | 1.09 ± 0.03 | 0.05 ± 0.00 | 1.04 ± 0.00 | 2.71 ± 0.05 | 1.06 ± 0.08 | 1.44 ± 0.02 |
| CE | 3600.00 | 49.72 ± 0.22 | 50.39 ± 1.08 | 41.22 ± 1.03 | 0.00 ± 0.00 | 0.09 ± 0.01 | 1.00 ± 0.01 | 0.04 ± 0.00 | 0.97 ± 0.03 | 2.36 ± 0.02 | 1.1 ± 0.03 | 1.40 ± 0.01 |
| A | t | Ds | AOP DPPH | AOP FRAP | TPC | trans-resv- 3-O-gluc | trans Resveratrol | Quercetin | Kaempferol |
|---|---|---|---|---|---|---|---|---|---|
| 90.000 | 1361.36 | 22 | 76.05 | 122.98 | 38.67 | 31.56 | 36.36 | 41.43 | 68.69 |
| 90.000 | 1364.32 | 22 | 76.07 | 123.06 | 38.69 | 31.73 | 36.36 | 41.53 | 68.69 |
| 90.000 | 1468.60 | 22 | 76.39 | 125.33 | 39.28 | 34.05 | 36.36 | 43.33 | 71.72 |
| 89.999 | 1313.80 | 22 | 75.71 | 121.66 | 38.37 | 30.57 | 36.36 | 40.64 | 67.68 |
| 90.000 | 1497.93 | 22 | 76.38 | 125.83 | 39.45 | 34.72 | 36.36 | 43.83 | 72.73 |
| A | t | Ds | Pt-3-Glucoside | Mv-3-Glucoside | Mv-3-acetyl gluc | Mv-3-p-coum gluc | Desirability |
|---|---|---|---|---|---|---|---|
| 90.00 | 1361.34 | 22 | 41.92 | 49.10 | 25.98 | 26.88 | 0.826 |
| 90.00 | 1364.32 | 22 | 41.97 | 49.10 | 25.90 | 26.88 | 0.826 |
| 90.00 | 1468.59 | 22 | 42.90 | 48.01 | 24.35 | 26.77 | 0.824 |
| 89.99 | 1313.79 | 22 | 41.52 | 49.43 | 26.49 | 26.88 | 0.824 |
| 90.00 | 1497.93 | 22 | 43.12 | 47.63 | 23.84 | 26.77 | 0.823 |
| A | t | Ds | AOP DPPH | AOP FRAP | TPC | trans-resv- 3-O-gluc | trans Resveratrol | Quercetin | Kaempferol |
|---|---|---|---|---|---|---|---|---|---|
| 90.00 | 1499.98 | 22 | 62.19 | 104.79 | 4.620 | 300.00 | 45.75 | 42.23 | 67.44 |
| 90.00 | 1467.48 | 22 | 62.20 | 104.33 | 3.54 | 300.00 | 44.68 | 42.03 | 67.44 |
| 86.17 | 1499.99 | 22 | 61.01 | 103.10 | 9.61 | 300.00 | 43.62 | 41.24 | 65.12 |
| 85.57 | 1499.98 | 22 | 60.83 | 102.81 | 10.31 | 300.00 | 43.62 | 41.04 | 65.12 |
| 81.49 | 1500.00 | 22 | 59.57 | 100.78 | 14.41 | 300.00 | 41.49 | 39.94 | 65.12 |
| A | t | Ds | Pt-3-Glucoside | Mv-3-Glucoside | Mv-3-acetyl gluc | Mv-3-p-coum gluc | Desirability |
|---|---|---|---|---|---|---|---|
| 90.00 | 1499.99 | 22 | 64.95 | 89.17 | 49.74 | 34.93 | 0.828 |
| 90.00 | 1467.48 | 22 | 64.33 | 88.03 | 49.04 | 34.50 | 0.826 |
| 86.17 | 1499.99 | 22 | 63.61 | 87.94 | 46.77 | 33.21 | 0.822 |
| 85.50 | 1499.98 | 22 | 63.40 | 87.73 | 46.34 | 33.00 | 0.821 |
| 81.49 | 1500.00 | 22 | 61.86 | 86.38 | 43.46 | 31.29 | 0.812 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T.; et al. Non-Thermal Ultrasonic Extraction of Polyphenolic Compounds from Red Wine Lees. Foods 2020, 9, 472. https://doi.org/10.3390/foods9040472
Dujmić F, Kovačević Ganić K, Ćurić D, Karlović S, Bosiljkov T, Ježek D, Vidrih R, Hribar J, Zlatić E, Prusina T, et al. Non-Thermal Ultrasonic Extraction of Polyphenolic Compounds from Red Wine Lees. Foods. 2020; 9(4):472. https://doi.org/10.3390/foods9040472
Chicago/Turabian StyleDujmić, Filip, Karin Kovačević Ganić, Duska Ćurić, Sven Karlović, Tomislav Bosiljkov, Damir Ježek, Rajko Vidrih, Janez Hribar, Emil Zlatić, Tihomir Prusina, and et al. 2020. "Non-Thermal Ultrasonic Extraction of Polyphenolic Compounds from Red Wine Lees" Foods 9, no. 4: 472. https://doi.org/10.3390/foods9040472