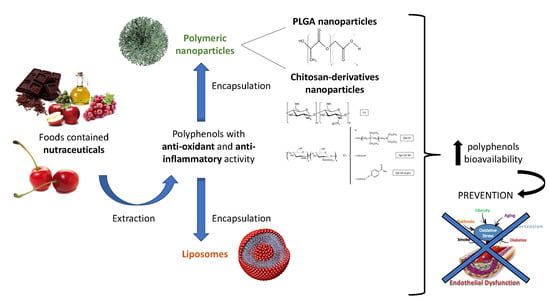
Antioxidant and Anti-Inflammatory Properties of Cherry Extract: Nanosystems-Based Strategies to Improve Endothelial Function and Intestinal Absorption
Abstract
:1. Introduction
2. Cardiovascular Diseases
3. Inflammation
4. Role of Oxidative Stress
Model for the Study of Endothelial Dysfunction
5. Nutraceutical Intervention
6. Polyphenols and Sweet Cherry (Prunus avium L.)
7. Nanotechnology in Nutraceutical
7.1. Nanoparticles Based on Chitosan Derivatives
7.2. Poly(Lactic-co-glycolic Acid) Nanoparticles
7.3. Liposomes
8. Intestinal Absorption
Triple Cell Co-Culture (Caco-2/HT29-MTX/Raji B) as a Model of Study
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kris-Etherton, P.; Eckel, R.H.; Howard, B.V.; St. Jeor, S.; Bazzarre, T.L. Benefits of a Mediterranean-Style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation 2001, 103, 1823–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Kelley, D.S.; Rasooly, R.; Jacob, R.A.; Kader, A.A.; Mackey, B.E. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J. Nutr. 2006, 136, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Fogli, S.; Zaino, C.; Stefanelli, F.; Breschi, M.C.; Di Colo, G. Synthesis, characterization and evaluation of thiolated quaternary ammonium-chitosan conjugates for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2009, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.; Avdonin, P.; Nadeev, A.; Zharkikh, I.; Jenkins, R. Reactive Oxygen Species in Pathogenesis of Atherosclerosis. Curr. Pharm. Des. 2014, 21, 1134–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Dejana, E.; Spagnuolo, R.; Bazzoni, G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb. Haemost. 2001, 86, 308–315. [Google Scholar]
- Yingshun, X.; Melendez, A.J. Secreted proinflammatory mediators in atherosclerosis: New insights and potential novel therapeutics applications. IJIB 2007, 1, 65–71. [Google Scholar]
- Understanding the Vasculature with the Help of HUVECs. Available online: https://www.promocell.com/in-the-lab/understanding-vasculature-help-huvecs/2017 (accessed on 21 July 2017).
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Patel, H.; Chen, J.; Das, K.C.; Kavdia, M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc. Diabetol. 2013, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Walshe, T.E.; Dela Paz, N.G.; D’Amore, P.A. The role of shear-induced transforming growth factor-β signaling in the endothelium. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2608–2617. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Jung, Y.; Kim, Y.; Jho, E.H.; Yoon, Y. LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci. Rep. 2017, 7, 41612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Hafizah, A.H.; Zaiton, Z.; Zulkhairi, A.; Ilham, A.M.; Anita, M.N.; Zaleha, A.M. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. J. Zhejiang Univ. Sci. B 2010, 11, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.L.; Liu, Y.; Liu, M.; Hu, H.; Pan, Y.; Fan, X.J.; Zou, W.W. Inhibition of hydrogen peroxide-induced human umbilical vein endothelial cells aging by allicin depends on Sirtuin1 activation. Med. Sci. Monit. 2017, 23, 563. [Google Scholar] [CrossRef] [Green Version]
- Felice, F.; Maragò, E.; Sebastiani, L.; Di Stefano, R. Apple juices from ancient Italian cultivars: A study on mature endothelial cells model. Fruits 2015, 70, 361–369. [Google Scholar] [CrossRef]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–732. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical—Definition and introduction. AAPS J. 2003, 5, 27–28. [Google Scholar] [CrossRef] [Green Version]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Ross, S. Functional foods: The Food and Drug Administration perspective. Am. J. Clin. Nutr. 2000, 71, 1735s–1738s. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Sivagnanam, G.; Xavier, R. Nutraceuticals as therapeutic agents: A Review. Res. J. Pharm. Technol. 2008, 1, 328–340. [Google Scholar]
- Zhao, C.N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.Y.; Li, H. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Imran, A.; Sharif, M.K.; Ahmad, R.S.; Xiao, H.; Imran, M.; Rsool, H.A. Black tea polyphenols: A mechanistic treatise. Crit. Rev. Food Sci. Nutr. 2014, 54, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Buttxs, M.S.; Qayyum, M.M.N.; Suleria, H.A.R. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Popular functional foods and nutraceuticals with lipid lowering activity and in relation to cardiovascular disease, dyslipidemia, and related complications: An overview. J. Food Bioact. 2018, 2, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, J.P.; Ryan, S.M.; Brayden, D.J. Oral delivery strategies for nutraceuticals: Delivery vehicles and absorption enhancers. Trends Food Sci. Technol. 2016, 53, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-γ-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Mitjans, M.; Vinardell, M.P. Cytoprotective Effects of Polyphenols against Oxidative Damage. Polyphen. Hum. Health Dis. 2014, 1, 275–288. [Google Scholar]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds: A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and Health: A Review. Crit. Rev. Food Sci. Nutr. 2010, 51, 1–12. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Ponde, A.; Hallmann, E.; Głowacka, A.; Rozpara, E. The Profile and Content of Polyphenols and Carotenoids in Local and Commercial Sweet Cherry Fruits (Prunus avium L.) and Their Antioxidant Activity In Vitro. Antioxidants 2019, 8, 534. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.F.; Guerriero, G.; Cai, G. Agrobiotechnology Goes Wild: Ancient Local Varieties as Sources of Bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.F.; Guerriero, G.; Cai, G. Functional Molecules in Locally-Adapted Crops: The Case Study of Tomatoes, Onions, and Sweet Cherry Fruits from Tuscany in Italy. Front. Plant Sci. 2018, 9, 1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, L.; De Lima, R.; De Oliveira Assumpção, C.; Prestes, J.; Denadai, B.S. Consumption of cherries as a strategy to attenuate exercise-induced muscle damage and inflammation in humans. Nutr. Hosp. 2015, 32, 1885–1893. [Google Scholar]
- Kelley, D.S.; Adkins, Y.; Reddy, A.; Woodhouse, L.R.; Mackey, B.E.; Erickson, K.L. Sweet Bing Cherries Lower Circulating Concentrations of Markers for Chronic Inflammatory Diseases in Healthy Humans. J. Nutr. 2013, 143, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus Cerasus, L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phytother. Res. 2019. [Google Scholar] [CrossRef]
- Lietava, J.; Beerova, N.; Klymenko, S.V.; Panghyova, E.; Varga, I.; Pechanova, O. Effects of Cornelian Cherry on Atherosclerosis and Its Risk Factors. Oxid. Med. Cell. Longev. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Robinson, J.L.; Hunter, J.M.; Reyes-Izquierdo, T.; Argumedo, R.; Brizuela-Bastien, J.; Keller, R.; Pietrzkowski, Z. Cognitive short- and long-term effects of coffee cherry extract in older adults with mild cognitive decline. Neuropsychol. Dev. Cognit. B Aging Neuropsychol. Cognit. 2019. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Effcacy of administering fruit-derived polyphenols to improve health biomarkers, exercise performance and related physiological responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Fratantonio, D.; Cimino, F.; Molonia, M.S.; Ferrari, D.; Saija, A.; Virgili, F.; Speciale, A. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Nie, X.; Shi, J.; Liu, Q.; Wang, Z.; Li, X.; Zhou, J.; Su, J.; Xue, M.; Chen, W.D.; et al. Quercetin inhibits LPS-induced inflammation and ox-LDL-induced lipid deposition. Front. Pharmacol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Console, L.; Giangregorio, N.; Cellamare, S.; Bolognino, I.; Palasciano, M.; Indiveri, C.; Incampo, G.; Campana, S.; Tonazzi, A. Human mitochondrial carnitine acylcarnitine carrier: Molecular target of dietary bioactive polyphenols from sweet cherry (Prunus avium L.). Chem. Biol. Interact. 2019, 307, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-Based Nanoparticles Containing Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beconcini, D.; Felice, F.; Zambito, Y.; Fabiano, A.; Piras, A.M.; Macedo, M.H.; Sarmento, B.; Di Stefano, R. Anti-Inflammatory Effect of Cherry Extract Loaded in Polymeric Nanoparticles: Relevance of Particle Internalization in Endothelial Cells. Pharmaceutics 2019, 11, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, M. Nanomedicine: Promising Tiny Machine for the Healthcare in Future-A Review. Oman Med. J. 2009, 24, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. General Aspects of Nanoemulsions and Their Formulation. In Nanoemulsions: Formulation, Applications, and Characterization; Academic Press: Cambridge, MS, USA, 2018; pp. 3–20. ISBN 9780128118399. [Google Scholar]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Punia, S.; Sandhu, K.S.; Kaur, M.; Siroha, A.K. Nanotechnology: A Successful Approach to Improve Nutraceutical Bioavailability. In Nanobiotechnology in Bioformulations; Springer: Cham, Switzerland, 2019; pp. 119–133. [Google Scholar]
- Kawashima, Y. Nanoparticulate systems for improved drug delivery. Adv. Drug Deliv. Rev. 2001, 47, 1–2. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Di Colo, G.; D’Onofrio, C.; Fausto, C.; Balbarini, A.; Di Stefano, R. Red grape skin and seeds polyphenols: Evidence of their protective effects on endothelial progenitor cells and improvement of their intestinal absorption. Eur. J. Pharm. Biopharm. 2012, 80, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; D’Onofrio, C.; Fabiano, A.; Balbarini, A.; Di Stefano, R. Delivery of natural polyphenols by polymeric nanoparticles improves the resistance of endothelial progenitor cells to oxidative stress. Eur. J. Pharm. Sci. 2013, 50, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. 2018, 130, 281–289. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Beconcini, D.; Fabiano, A.; Di Stefano, R.; Macedo, M.H.; Felice, F.; Zambito, Y.; Sarmento, B. Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles. Int. J. Mol. Sci. 2019, 20, 1759. [Google Scholar] [CrossRef] [Green Version]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A successful tool to increase solubility, stability and optimise bioefficacy of natural constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef]
- Sinico, C.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Bilia, A.R.; Vincieri, F.F. Liposomes as carriers for verbascoside: Stability and skin permeation studies. J. Liposome Res. 2008, 18, 83–90. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as potential drug carrier systems for drug delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014. [Google Scholar]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risaliti, L. Nanocarriers for the Oral and Topical Delivery of Natural Compounds. Ph.D. Thesis, University of Florence, Florence, Italy, 2019. [Google Scholar]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Fu, L.; Hussain, Z.; Huang, D.; Zhu, S. Enhancement of storability and antioxidant systems of sweet cherry fruit by nitric oxide-releasing chitosan nanoparticles (GSNO-CS NPs). Food Chem. 2019, 285, 10. [Google Scholar] [CrossRef]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef]
- Peniche, H.; Peniche, C. Chitosan nanoparticles: A contribution to nanomedicine. Polym. Int. 2011, 60, 883–889. [Google Scholar] [CrossRef]
- Kerch, G. The potential of chitosan and its derivatives in prevention and treatment of Age-related diseases. Mar. Drugs 2015, 13, 2158–2182. [Google Scholar] [CrossRef] [Green Version]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotzé, A.F.; Lueßen, H.L.; De Leeuw, B.J.; De Boer, B.G.; Coos Verhoef, J. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J. Controll. Release 1998, 51, 35–46. [Google Scholar] [CrossRef]
- Kotzé, A.F.; De Leeuw, B.J.; Lueßen, H.L.; De Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: In vitro evaluation in Caco-2 cell monolayers. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- Ylenia, Z.; di Colo, G. Thiolated quaternary ammonium–chitosan conjugates for enhanced precorneal retention, transcorneal permeation and intraocular absorption of dexamethasone. Eur. J. Pharm. Biopharm. 2010, 75, 194–199. [Google Scholar]
- Fabiano, A.; Mattii, L.; Braca, A.; Felice, F.; Di Stefano, R.; Zambito, Y. Nanoparticles based on quaternary ammonium-chitosan conjugate: A vehicle for oral administration of antioxidants contained in red grapes. J. Drug Deliv. Sci. Technol. 2016, 32, 291–297. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Hembram, K.C.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314. [Google Scholar] [CrossRef]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef]
- Ba, C.; Fu, Y.; Niu, F.; Wang, M.; Jin, B.; Li, Z.; Chen, G.; Zhang, H.; Li, X. Effects of environmental stresses on physiochemical stability of β-carotene in zein-carboxymethyl chitosan-tea polyphenols ternary delivery system. Food Chem. 2020, 311, 125878. [Google Scholar] [CrossRef]
- Khan, M.A.; Yue, C.; Fang, Z.; Hu, S.; Cheng, H.; Bakry, A.M.; Liang, L. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J. Food Eng. 2019, 258, 4553. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Controll. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, A.K.; Raj, V.; Rai, A.; Keshari, A.K.; Kumar, D.; Maity, B.; Prakash, A.; Maiti, S.; Saha, S. Poly(lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid for improved treatment of hepatic cancer: Characterization, in vitro and in vivo evaluations. Int. J. Nanomed. 2018, 13, 975. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.M.; Hill, L.E.; Figueiredo, E.; Gomes, C.L. Delivery of phytochemicals of tropical fruit by-products using poly (DL-lactide-co-glycolide) (PLGA) nanoparticles: Synthesis, characterization, and antimicrobial activity. Food Chem. 2014, 165, 362–370. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Controll. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Tahara, K.; Sakai, T.; Yamamoto, H.; Takeuchi, H.; Hirashima, N.; Kawashima, Y. Improved cellular uptake of chitosan-modified PLGA nanospheres by A549 cells. Int. J. Pharm. 2009, 382, 198–204. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Abd-Rabou, A.A.; Abdalla, A.M.; Ali, N.A.; Zoheir, K.M.A. Moringa oleifera root induces cancer apoptosis more effectively than leave nanocomposites and its free counterpart. APJCP 2017, 18, 2141–2149. [Google Scholar]
- Doolaanea, A.A.; Mansor, N.I.; Mohd Nor, N.H.; Mohamed, F. Co-encapsulation of Nigella sativa oil and plasmid DNA for enhanced gene therapy of Alzheimers disease. J. Microencapsul. 2016, 33, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Gupta, R.; Gulati, M.; Singh, S.K.; Khursheed, R.; Gupta, M. The Why, Where, Who, How, and What of the vesicular delivery systems. Adv. Colloid Interface Sci. 2019, 271, 101985. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Hill, M.W.; Miller, N.G.A. Preparation and use of liposomes as models of biological membranes. In Methods in Membrane Biology; Springer: Boston, MA, USA, 1974; pp. 1–68. [Google Scholar]
- Deamer, D.; Bangham, A.D. Large volume liposomes by an ether vaporization method. Biochim. Biophys. Acta Biomembr. 1976, 443, 629–634. [Google Scholar] [CrossRef]
- Gabizon, A.; Chisin, R.; Amselem, S.; Druckmann, S.; Cohen, R.; Goren, D.; Fromer, I.; Peretz, T.; Sulkes, A.; Barenholz, Y. Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br. J. Cancer 1991, 64, 1125–1132. [Google Scholar] [CrossRef] [Green Version]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on nature: The role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, P.A.; Yokota, D.; Foglio, M.A.; Rodrigues, R.A.F.; Pinho, S.C. Liposomes incorporating essential oil of Brazilian cherry (Eugenia uniflora L.): Characterization of aqueous dispersions and lyophilized formulations. J. Microencapsul. 2010, 27, 416–425. [Google Scholar] [CrossRef]
- Akgün, D.; Gültekin-Özgüven, M.; Yücetepe, A.; Altin, G.; Gibis, M.; Weiss, J.; Özçelik, B. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocoll. 2020, 101, 105532. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef]
- Araújo, F.; das Neves, J.; Martins, J.P.; Granja, P.L.; Santos, H.A.; Sarmento, B. Functionalized materials for multistage platforms in the oral delivery of biopharmaceuticals. Prog. Mater. Sci. 2017, 89, 306–344. [Google Scholar] [CrossRef]
- Angela, M. The effect of gastro-intestinal mucus on drug absorption. Adv. Drug Deliv. Rev. 1993, 11, 201–220. [Google Scholar]
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Hoffer, M.; Werle, M.; Bernkop-Schnürch, A. Improved synthesis and in vitro characterization of chitosan–thioethylamidine conjugate. Biomat 2006, 27, 127–135. [Google Scholar] [CrossRef]
- Otero, J.; García-Rodríguez, A.; Cano-Sarabia, M.; Maspoch, D.; Marcos, R.; Cortés, P.; Llagostera, M. Biodistribution of liposome-encapsulated bacteriophages and their transcytosis during oral phage therapy. Front. Microbiol. 2019, 10, 689. [Google Scholar] [CrossRef] [Green Version]
- Belubbi, T.; Shevade, S.; Dhawan, V.; Sridhar, V.; Majumdar, A.; Nunes, R.; Araújo, F.; Sarmento, B.; Nagarsenker, K.; Steiniger, F.; et al. Lipid Architectonics for Superior Oral Bioavailability of Nelfinavir Mesylate: Comparative in vitro and in vivo Assessment. AAPS PharmSciTech 2018, 19, 3584–3598. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beconcini, D.; Felice, F.; Fabiano, A.; Sarmento, B.; Zambito, Y.; Di Stefano, R. Antioxidant and Anti-Inflammatory Properties of Cherry Extract: Nanosystems-Based Strategies to Improve Endothelial Function and Intestinal Absorption. Foods 2020, 9, 207. https://doi.org/10.3390/foods9020207
Beconcini D, Felice F, Fabiano A, Sarmento B, Zambito Y, Di Stefano R. Antioxidant and Anti-Inflammatory Properties of Cherry Extract: Nanosystems-Based Strategies to Improve Endothelial Function and Intestinal Absorption. Foods. 2020; 9(2):207. https://doi.org/10.3390/foods9020207
Chicago/Turabian StyleBeconcini, Denise, Francesca Felice, Angela Fabiano, Bruno Sarmento, Ylenia Zambito, and Rossella Di Stefano. 2020. "Antioxidant and Anti-Inflammatory Properties of Cherry Extract: Nanosystems-Based Strategies to Improve Endothelial Function and Intestinal Absorption" Foods 9, no. 2: 207. https://doi.org/10.3390/foods9020207