Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System
Abstract
:1. Introduction
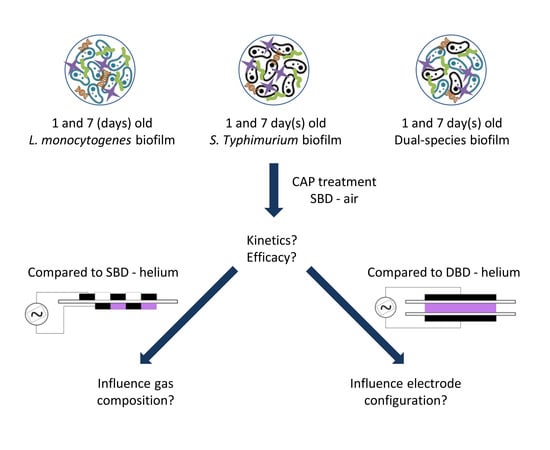
2. Materials and Methods
2.1. Experimental Design
2.2. Microorganism and Pre-Culture Conditions
2.3. Biofilm Development Conditions
2.4. CAP Equipment and Biofilm Inactivation Procedure
2.5. Quantification of Biofilm Cell Density by Means of Viable Plate Counts
2.6. Modelling, Parameter Estimation, and Statistical Analysis
3. Results and Discussion
3.1. Kinetics Obtained Following Biofilm Inactivation with an Air-Based CAP System
3.1.1. General Observations
3.1.2. Influence of the Biofilm Age
3.1.3. Influence Biofilm Type
3.1.4. Influence Population Type within Dual-Species Biofilm
3.1.5. Induction of Sub-Lethal Injury
3.2. Comparison between the Air-Based and Helium-Operated CAP System
3.2.1. General Observations
3.2.2. Differences in Inactivation Kinetics between SBD-air and SBD-helium
3.2.3. Differences in Inactivation Kinetics between SBD-air and DBD-helium
3.2.4. Induction of Sub-Lethal Injury
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakke, R.; Trulear, M.G.; Robinson, J.A.; Characklis, W.G. Activity of Pseudomonas aeruginosa in biofilms: Steady state. Biotechnol. Bioeng. 1984, 26, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 9, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.C.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Barry, D.M.; Kanematsu, H. Cooling Water. In Biofilm and Materials Science; Kanematsu, H., Barry, D.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 79–84. [Google Scholar]
- Javaherdashti, R. Corrosion and Biofilm. In Biofilm and Materials Science; Kanematsu, H., Barry, D.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 69–78. [Google Scholar]
- Gómez-López, V.M. Decontamination of Fresh and Minimally Processed Produce, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef]
- Srey, S.; Park, S.Y.; Jahid, I.K.; Ha, S.-D. Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Res. Int. 2014, 62, 484–491. [Google Scholar] [CrossRef]
- Ziuzina, D.; Han, L.; Cullen, P.J.; Bourke, P. Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli. Int. J. Food Microbiol. 2015, 210, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Govaert, M.; Smet, C.; Baka, M.; Ećimović, B.; Walsh, J.L.; Van Impe, J. Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as function of biofilm age. Appl. Sci. 2018, 8, 2702. [Google Scholar] [CrossRef] [Green Version]
- Govaert, M.; Smet, C.; Vergauwen, L.; Ećimović, B.; Walsh, J.L.; Baka, M.; Van Impe, J. Influence of plasma characteristics on the efficacy of Cold Atmospheric Plasma (CAP) for inactivation of Listeria monocytogenes and Salmonella Typhimurium biofilms. IFSET 2019, 52, 376–386. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Walsh, J.L.; Van Impe, J. Dual-species model biofilm consisting of Listeria monocytogenes and Salmonella Typhimurium: Development and inactivation with Cold Atmospheric Plasma (CAP). Front. Microbiol. 2019, 10, 2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Kudra, T.; Mujumdar, A.S. Advanced Drying Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Banu, M.S.; Sasikala, P.; Dhanapal, A.; Kavitha, V.; Yazhini, G.; Rajamani, L. Cold plasma as a novel food processing technology. IJETED 2012, 4, 803–818. [Google Scholar]
- Fernández, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Food Res. Int. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Lu, H.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial Inactivation by High Voltage Atmospheric Cold Plasma: Influence of Process Parameters and Effects on Cell Leakage and DNA. J. Appl. Microbiol. 2013, 116, 784–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [Green Version]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. On the penetration depth of reactive oxygen and nitrogen species generated by a plasma jet through real biological tissue. Phys. Plasmas 2017, 24, 073506. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Baka, M.; Janssens, T.; Van Impe, J. Influence of incubation conditions on the formation of model biofilms by Listeria monocytogenes and Salmonella Typhimurium on abiotic surfaces. J. Appl. Microbiol. 2018, 125, 1890–1990. [Google Scholar] [CrossRef]
- Noriega, E.; Velliou, E.; Van Derlinden, E.; Mertens, L.; Van Impe, J.F. Effect of cell immobilization on heat-induced sublethal injury of Escherichia coli, Salmonella Typhimurium and Listeria innocua. Food Microbiol. 2013, 36, 355–364. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Busch, S.V.; Donnelly, C.W. Development of a repair-enrichment broth for resuscitation of heat-injured Listeria monocytogenes and Listeria innocua. Appl. Envrion. Microbiol. 1992, 58, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Anwar, H.; Strap, J.L.; Costerton, J.W. Establishment of Aging Biofilms: Possible Mechanism of Bacterial Resistance to Antimicrobial Therapy. Antimicrob. Agents Chemother. 1992, 36, 1347–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Oral anaerobes cannot survive oxygen stress without interacting with facultative/aerobic species as a microbial community. Lett. Appl. Microbiol. 1997, 25, 385–387. [Google Scholar] [CrossRef]
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wu, H.; Høiby, N.; Molin, S.; Song, Z. Current understanding of multi-species biofilms. Int. J. Oral Sci. 2011, 3, 74–81. [Google Scholar] [CrossRef]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S.J. Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- Ragni, L.; Berardinelli, A.; Vannini, L.; Montanari, C.; Sirri, F.; Guerzoni, M.E.; Guarnieri, A. Non-thermal atmospheric gas plasma device for surface decontamination of shell eggs. J. Food Eng. 2010, 100, 125–132. [Google Scholar] [CrossRef]
- Hasan, M.I.; Walsh, J.L. Influence of gas flow velocity on the transport of chemical species in an atmospheric pressure air plasma discharge. Appl. Phys. Lett. 2017, 110, 134102. [Google Scholar] [CrossRef] [Green Version]
- Dickenson, A.; Britun, N.; Nikiforov, A.; Leys, C.; Hasan, M.I.; Walsh, J.L. The generation and transport of reactive nitrogen species from a low temperature atmospheric pressure air plasma source. Phys. Chem. Chem. Phys. 2018, 20, 28499–28510. [Google Scholar] [CrossRef]
- Murakami, T.; Niemi, K.; Gans, T.; O’Connell, D.; Graham, W.G. Afterglow chemistry of atmospheric-pressure helium–oxygen plasmas with humid air impurity. Plasma Sources Sci. Technol. 2014, 23, 025005. [Google Scholar] [CrossRef]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined Effects of Long-Living Chemical Species during Microbial Inactivation Using Atmospheric Plasma-Treated Water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in Water Electro-Sprayed through Transient Spark Discharge in Air and their Bactericidal Effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of Non-Thermal Atmospheric Pressure Plasma with Solid and Liquid Food Systems: A Review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Verheyen, D.; Walsh, J.L.; Van Impe, J. Combined effect of Cold Atmospheric Plasma and hydrogen peroxide treatment on mature Listeria monocytogenes and Salmonella Typhimurium biofilms. Front. Microbiol. 2019, 10, 2674. [Google Scholar] [CrossRef] [Green Version]




| Single-Species L. monocytogenes 1-day-old | Single-Species L. monocytogenes 7-day-old | Single-Species S. Typhimurium 1 Day Old | Single-Species S. Typhimurium 7-day-old | Dual-Species 1-day-old | Dual-Species 7-day-old | |
|---|---|---|---|---|---|---|
| 23 Log10 N0 general medium 15 4 (log(CFU/cm2)) | b 7.04 ± 0.23 b A 1 | a 6.32 ± 0.11 b A | a 5.96 ± 0.17 a A 1 | a 5.69 ± 0.18 a A | ab 5.84 ± 0.21 a A | ac 5.69 ± 0.16 a A |
| 23 Log10 N0 PALCAM medium 15 4 (log(CFU/cm2)) | b 6.99 ± 0.25 b A 1 | a 6.18 ± 0.10 b A | NA | NA | aa 5.00 ± 0.23 a A | aa 4.79 ± 0.16 a A |
| 23 Log10 N0 XLD medium 15 4 (log(CFU/cm2)) | NA | NA | a 5.80 ± 0.20 a A 1 | a 5.31 ± 0.33 a A | ab 5.56 ± 0.30 a A | ab 5.11 ± 0.13 a A |
| 23kmax general medium 15 4 (1/min) | b 0.705 ± 0.192 a A 1 | a 0.195 ± 0.018 a A | a 0.605 ± 0.116 a A 1 | a 0.396 ± 0.085 a A | aa 0.529 ± 0.131 a A | bb 2.002 ± 0.661 b B |
| 23kmax PALCAM medium 15 4 (1/min) | b 0.826 ± 0.265 a A 1 | a 0.199 ± 0.017 a A | NA | NA | aa 0.650 ± 0.227 a A | aa 0.517 ± 0.159 b A |
| 23kmax XLD medium 15 4 (1/min) | NA | NA | a 0.663 ± 0.114 a A 1 | a 0.722 ± 0.195 a A | aa 0.618 ± 0.160 a A | bb 1.702 ± 0.482 b A |
| 23 Log10 Nres general medium 15 4 (log(CFU/cm2)) | 4.57 ± 0.22 b B 1 | / | a 3.46 ± 0.17 a B 1 | a 3.25 ± 0.22 a A | ab 3.44 ± 0.22 a A | bc 4.17 ± 0.10 b B |
| 23 Log10 Nres PALCAM medium 15 4 (log(CFU/cm2)) | 4.52 ± 0.23 b B 1 | / | NA | NA | ab 3.01 ± 0.21 a A | aa 3.13 ± 0.16 B |
| 23 Log10 Nres XLD medium 15 4 (log(CFU/cm2)) | NA | NA | a 2.57 ± 0.22 a B 1 | a 1.96 ± 0.35 a A | aa 2.41 ± 0.32 a A | bb 3.64 ± 0.09 b B |
| 23 Log-reduction general medium 15 4 (log(CFU/cm2)) | a 2.47 ± 0.32 a A 1 | ≈ a 2.53 ± 0.12 b A | a 2.50 ± 0.24 a A 1 | a 2.44 ± 0.28 b A | ba 2.40 ± 0.31 a A | aa 1.51 ± 0.19 a A |
| 23 Log-reduction PALCAM medium 15 4 (log(CFU/cm2)) | a 2.47 ± 0.34 a A 1 | ≈ a 2.60 ± 0.11 b A | NA | NA | aa 1.98 ± 0.30 a A | aa 1.66 ± 0.23 a A |
| 23 Log-reduction XLD medium 15 4 (log(CFU/cm2)) | NA | NA | a 3.23 ± 0.30 a A 2 | a 3.34 ± 0.49 b A | bb 3.15 ± 0.44 a A | aa 1.47 ± 0.16 a A |
| RMSE general medium (/) | 0.653 | 0.396 | 0.490 | 0.532 | 0.615 | 0.389 |
| RMSE PALCAM medium (/) | 0.716 | 0.371 | NA | NA | 0.625 | 0.464 |
| RMSE XLD medium (/) | NA | NA | 0.602 | 0.988 | 0.878 | 0.331 |
| Single-Species L. monocytogenes 1-day-old | Single-Species S. Typhimurium 1-day-old | |
|---|---|---|
| Log10 N0 general medium 4 (log(CFU/cm2)) | 7.17 ± 0.07 1 | 6.49 ± 0.07 2 |
| Log10 N0 PALCAM medium 4 (log(CFU/cm2)) | 7.16 ± 0.07 1 | NA |
| Log10 N0 XLD medium 4 (log(CFU/cm2)) | NA | 6.19 ± 0.07 2 |
| kmax general medium 4 (1/min) | 2.343 ± 0.577 2 | 1.747 ± 0.351 2 |
| kmax PALCAM medium 4 (1/min) | 2.771 ± 0.491 2 | NA |
| kmax XLD medium 4 (1/min) | NA | 2.876 ± 0.584 2 |
| Log10 Nres general medium 4 (log(CFU/cm2)) | 5.22 ± 0.11 2 | 4.29 ± 0.11 2 |
| Log10 Nres PALCAM medium 4 (log(CFU/cm2)) | 4.77 ± 0.11 1 | NA |
| Log10 Nres XLD medium 4 (log(CFU/cm2)) | NA | 3.86 ± 0.11 2 |
| Log-reduction general medium 4 (log(CFU/cm2)) | 1.95 ± 0.14 1 | 2.19 ± 0.13 1 |
| Log-reduction PALCAM medium 4 (log(CFU/cm2)) | 2.40 ± 0.13 1 | NA |
| Log-reduction XLD medium 4 (log(CFU/cm2)) | NA | 2.33 ± 0.13 1 |
| RMSE general medium (/) | 0.432 | 0.396 |
| RMSE PALCAM medium (/) | 0.394 | NA |
| RMSE XLD medium (/) | NA | 0.425 |
| Single-Species L. monocytogenes 1-day-old | Single-Specie L. monocytogenes 7-day-old | Single-Species S. Typhimurium 1-day-old | Single-Species S. Typhimurium 7-day-old | Dual-Species 1-day-old | Dual-Species 7-day-old | |
|---|---|---|---|---|---|---|
| Log10 N0 general medium 5 (log(CFU/cm2)) | 7.15 ± 0.07 A | 6.15 ± 0.22 A | 6.43 ± 0.07 B | 5.86 ± 0.23 A | 5.95 ± 0.15 A | 5.98 ± 0.13 A |
| Log10 N0 PALCAM medium 5 (log(CFU/cm2)) | 7.15 ± 0.07 A | 6.22 ± 0.24 A | NA | NA | 5.30 ± 0.21 A | 5.09 ± 0.19 A |
| Log10 N0 XLD medium 5 (log(CFU/cm2)) | NA | NA | 6.14 ± 0.09 A | 5.40 ± 0.25 A | 5.63 ± 0.20 A | 5.69 ± 0.24 B |
| kmax general medium 5 (1/min) | 1.265 ± 0.142 B | 0.253 ± 0.057 A | 1.308 ± 0.154 B | 2.730 ± 0.785 B | 0.748 ± 0.117 A | 0.618 ± 0.072 A |
| kmax PALCAM medium 5 (1/min) | 1.735 ± 0.172 B | 0.278 ± 0.078 A | NA | NA | 1.118 ± 0.199 A | 0.756 ± 0.121 A |
| kmax XLD medium 5 (1/min) | NA | NA | 1.534 ± 0.198 B | 2.598 ± 0.687 B | 1.148 ± 0.233 B | 1.542 ± 0.331 A |
| Log10 Nres general medium 5 (log(CFU/cm2)) | 3.50 ± 0.13 A | 2.68 ± 1.71 | 2.97 ± 0.13 A | 3.47 ± 0.16 A | 3.00 ± 0.17 A | 2.65 ± 0.16 A |
| Log10 Nres PALCAM medium 5 (log(CFU/cm2)) | 2.94 ± 0.13 A | 3.50 ± 0.56 | NA | NA | 1.51 ± 0.23 A | 1.12 ± 0.24 A |
| Log10 Nres XLD medium 5 (log(CFU/cm2)) | NA | NA | 2.13 ± 0.16 A | 2.68 ± 0.17 B | 2.30 ± 0.20 A | 1.80 ± 0.23 A |
| Log-reduction general medium 5 (log(CFU/cm2)) | 3.64 ± 0.15 B | 3.47 ± 1.72 A | 3.46 ± 0.14 B | 2.38 ± 0.28 A | 2.95 ± 0.23 A | 3.32 ± 0.21 B |
| Log-reduction PALCAM medium 5 (log(CFU/cm2)) | 4.22 ± 0.15 B | 2.72 ± 0.61 A | NA | NA | 3.79 ± 0.31 B | 3.97 ± 0.31 B |
| Log-reduction XLD medium 5 (log(CFU/cm2)) | NA | NA | 4.01 ± 0.18 B | 2.72 ± 0.30 A | 3.33 ± 0.28 A | 3.89 ± 0.33 B |
| RMSE general medium (/) | 0.435 | 0.733 | 0.420 | 0.571 | 0.500 | 0.439 |
| RMSE PALCAM medium (/) | 0.433 | 0.762 | NA | NA | 0.704 | 0.658 |
| RMSE XLD medium (/) | NA | NA | 0.547 | 0.626 | 0.646 | 0.784 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govaert, M.; Smet, C.; Graeffe, A.; L. Walsh, J.; Van Impe, J.F.M. Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System. Foods 2020, 9, 157. https://doi.org/10.3390/foods9020157
Govaert M, Smet C, Graeffe A, L. Walsh J, Van Impe JFM. Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System. Foods. 2020; 9(2):157. https://doi.org/10.3390/foods9020157
Chicago/Turabian StyleGovaert, Marlies, Cindy Smet, Annika Graeffe, James L. Walsh, and Jan F. M. Van Impe. 2020. "Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System" Foods 9, no. 2: 157. https://doi.org/10.3390/foods9020157