Food Protein Sterylation: Chemical Reactions between Reactive Amino Acids and Sterol Oxidation Products under Food Processing Conditions
Abstract
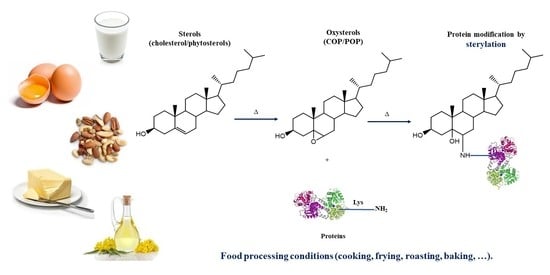
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Incubations of Amino Acids with Sterols/Oxysterols
2.3. Kinetic Studies of the Reaction between Cholesterol 5α,6α-Epoxide and Nα-Benzoylglycyl-l-Lysine
2.3.1. Effect of Time
2.3.2. Effect of Temperature
2.3.3. Effect of Concentrations
2.3.4. Analyses of Samples
2.4. GC-FID Analyses of Sterols and Cholesterol 5α,6α-Epoxide
2.5. HPLC Diode Array Detector (DAD) Analysis
2.6. HPLC-MS/MS Analysis
2.7. Statistical Analysis
3. Results
3.1. Identification of Reaction Products between Cholesterol 5α,6α-Epoxide and Amino Acid Side Chains
3.1.1. TLC Analyses
3.1.2. HPLC-DAD Analyses
3.1.3. HPLC-ESI-MS/MS Analyses
3.2. Kinetic Studies of the Reaction between Cholesterol 5α,6α-Epoxide and Nα-Benzoylglycyl-l-Lysine
3.2.1. Validation of the Analytical Methods
GC-FID Method Validation
HPLC-DAD Method Validation
3.2.2. Effect of Time
3.2.3. Effect of Temperature
3.2.4. Effect of Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography |
| GC | Gas chromatography |
| FID | Flame ionization detector |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| ESI | Electrospray ionization |
| Q-TOF MS | Quadrupole time-of-flight mass spectrometry |
References
- Maldonado-Pereira, L.; Schweiss, M.; Barnaba, C.; Medina-Meza, I.G. The role of cholesterol oxidation products in food toxicity. Food Chem. Toxicol. 2018, 118, 908–939. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nzekoue, F.K.; Caprioli, G.; Ricciutelli, M.; Cortese, M.; Alesi, A.; Vittori, S.; Sagratini, G. Development of an innovative phytosterol derivatization method to improve the HPLC-DAD analysis and the ESI-MS detection of plant sterols/stanols. Food Res. Int. 2020, 131, 108998. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, L.; Belwal, T.; Li, L.; Luo, Z. Phytosterols extraction from hickory (Carya cathayensis Sarg.) husk with a green direct citric acid hydrolysis extraction method. Food Chem. 2020, 315, 126217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Huang, K.C.; Su, C.H. Green process for the preparation of phytosterol esters: Microwave-mediated noncatalytic synthesis. Chem. Eng. 2020, 382, 122796. [Google Scholar] [CrossRef]
- Kamgang Nzekoue, F.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent coffee grounds: A potential commercial source of phytosterols. Food Chem. 2020, 325, 126836. [Google Scholar] [CrossRef]
- Lin, Y.; Koppenol, W.P.; Knol, D.; Vermeer, M.A.; Hiemstra, H.; Friedrichs, S.; Lutjohann, D.; Trautwein, E.A. Serum Concentration of Plant Sterol Oxidation Products (POP) Compared to Cholesterol Oxidation Products (COP) after Intake of Oxidized Plant Sterols: A Randomised, Placebo-Controlled, Double-Blind Dose–Response Pilot Study. Nutrients 2019, 11, 2319. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Lu, B. How do oxyphytosterols affect human health? Trends Food Sci. Technol. 2018, 79, 148–159. [Google Scholar] [CrossRef]
- Massimo, L.; Ginevra, L.B. Phytosterols and phytosterol oxides in Bronte’s Pistachio (Pistacia vera L.) and in processed pistachio products. Eur. Food Res. Technol. 2020, 246, 307–314. [Google Scholar] [CrossRef]
- Kasprzak, M.; Rudzińska, M.; Kmiecik, D.; Przybylski, R.; Olejnik, A. Acyl moiety and temperature affects thermo-oxidative degradation of steryl esters. Cytotoxicity of the degradation products. Food Chem. Toxicol. 2020, 136, 111074. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol oxidation products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur. J. Lipid Sci. Technol. 2016, 118, 1423–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef] [PubMed]
- Škulj, S.; Vazdar, K.; Margetić, D.; Vazdar, M. Revisited Mechanism of Reaction between a Model Lysine Amino Acid Side Chain and 4-Hydroxynonenal in Different Solvent Environments. J. Org. Chem. 2018, 84, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Globisch, M.; Schindler, M.; Kreßler, J.; Henle, T. Studies on the reaction of trans-2-heptenal with peanut proteins. J. Agric. Food Chem. 2014, 62, 8500–8507. [Google Scholar] [CrossRef] [PubMed]
- Globisch, M.; Kaden, D.; Henle, T. 4-Hydroxy-2-nonenal (4-HNE) and its lipation product 2-pentylpyrrole lysine (2-PPL) in peanuts. J. Agric. Food Chem. 2015, 63, 5273–5281. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Van Neerven, J.; Savelkoul, H. Food processing: The influence of the Maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Toda, M.; Hellwig, M.; Henle, T.; Vieths, S. Influence of the Maillard reaction on the allergenicity of food proteins and the development of allergic inflammation. Curr. Allergy Asthma Rep. 2019, 19, 4. [Google Scholar] [CrossRef]
- Taş, N. Investigation of Chemical Reactions in Hazelnut Induced By Roasting. Ph.D. Thesis, Hacettepe University, Ankara, Turkey, 2017. [Google Scholar]
- Zhao, Y.; Yang, B.; Xu, T.; Wang, M.; Lu, B. Photooxidation of phytosterols in oil matrix: Effects of the light, photosensitizers and unsaturation degree of the lipids. Food Chem. 2019, 288, 162–169. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Menéndez-Carreño, M.; Baris, R.; Janssen, H.G.; Trautwein, E.A. Oxidation of sitosterol and campesterol in foods upon cooking with liquid margarines without and with added plant sterol esters. Food Chem. 2018, 241, 387–396. [Google Scholar] [CrossRef]
- Menéndez-Carreño, M.; Knol, D.; Janssen, H.G. Development and validation of methodologies for the quantification of phytosterols and phytosterol oxidation products in cooked and baked food products. J. Chromatogr. A 2016, 1428, 316–325. [Google Scholar] [CrossRef]
- Treibmann, S.; Hellwig, A.; Hellwig, M.; Henle, T. Lysine-derived protein-bound Heyns compounds in bakery products. J. Agric. Food Chem. 2017, 65, 10562–10570. [Google Scholar] [CrossRef] [PubMed]
- Globisch, M.; Deuber, M.; Henle, T. Identification and Quantitation of the Lipation Product 2-Amino-6-(3-methylpyridin-1-ium-1-yl) hexanoic Acid (MP-Lysine) in Peanuts. J. Agric. Food Chem. 2016, 64, 6605–6612. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.B.; Sousa, B.C.; Pitt, A.R.; Spickett, C.M. A mass spectrometry approach for the identification and localization of small aldehyde modifications of proteins. Arch. Biochem. Biophys. 2018, 646, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kentala, H.; Weber-Boyvat, M.; Olkkonen, V.M. OSBP-related protein family: Mediators of lipid transport and signaling at membrane contact sites. Int. Rev. Cell Mol. Biol. 2016, 321, 299–340. [Google Scholar] [PubMed]
- Liu, H.; Huang, S. Role of oxysterol-binding protein-related proteins in malignant human tumours. World J. Clin. Cases 2020, 8, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Moumin, D.S.; Ciulla, D.A.; Owen, T.S.; Mancusi, R.A.; Giner, J.-L.; Wang, C.; Callahan, B.P. Protein–nucleic acid conjugation with sterol linkers using Hedgehog autoprocessing. Bioconj. Chem. 2019, 30, 2799–2804. [Google Scholar] [CrossRef]
- Sobhy, R.; Zhan, F.; Mekawi, E.; Khalifa, I.; Liang, H.; Li, B. The noncovalent conjugations of bovine serum albumin with three structurally different phytosterols exerted antiglycation effects: A study with AGEs-inhibition, multispectral, and docking investigations. Bioorg. Chem. 2020, 94, 103478. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Garcia-Llatas, G.; Sánchez-Siles, L.M.; Barberá, R.; Lagarda, M.J. Safe intake of a plant sterol-enriched beverage with milk fat globule membrane: Bioaccessibility of sterol oxides during storage. J. Food Compos. Anal. 2018, 68, 111–117. [Google Scholar] [CrossRef]









| Compounds | Linear Range (mM) | Regression Line | R2 | Reproducibility (RSD %, n = 5) | Sensitivity | ||
|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | LOD (µM) | LOQ (μM) | ||||
| Cholesterol 5α,6α-epoxide | 0.025–1.25 | Y = 6.6399x − 0.1027 | 0.9992 | 0.6 | 0.8 | 4.5 | 15 |
| Nα-benzoylglycyl-l-lysine | 0.05–1.0 | Y = 8908.6x + 417.1 | 0.9953 | 1.9 | 3.9 | 0.3 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamgang Nzekoue, F.; Henle, T.; Caprioli, G.; Sagratini, G.; Hellwig, M. Food Protein Sterylation: Chemical Reactions between Reactive Amino Acids and Sterol Oxidation Products under Food Processing Conditions. Foods 2020, 9, 1882. https://doi.org/10.3390/foods9121882
Kamgang Nzekoue F, Henle T, Caprioli G, Sagratini G, Hellwig M. Food Protein Sterylation: Chemical Reactions between Reactive Amino Acids and Sterol Oxidation Products under Food Processing Conditions. Foods. 2020; 9(12):1882. https://doi.org/10.3390/foods9121882
Chicago/Turabian StyleKamgang Nzekoue, Franks, Thomas Henle, Giovanni Caprioli, Gianni Sagratini, and Michael Hellwig. 2020. "Food Protein Sterylation: Chemical Reactions between Reactive Amino Acids and Sterol Oxidation Products under Food Processing Conditions" Foods 9, no. 12: 1882. https://doi.org/10.3390/foods9121882