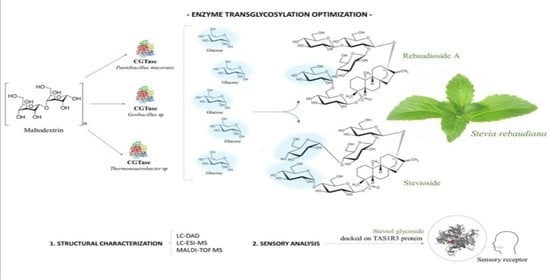
Transglycosylation of Steviol Glycosides and Rebaudioside A: Synthesis Optimization, Structural Analysis and Sensory Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design of Transglucosylation of Steviol Glycosides
2.3. Liquid Chromatography Coupled to a Diode-Array Detector (LC-DAD)
2.4. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS)
2.6. Isolation of Modified Steviol Glycosides and Rebaudioside A for Sensory Analysis
2.7. Sensory Analysis
3. Results
3.1. Optimization of Steviol Glycosides and Rebaudioside: A Transglucosylation Parameters by Response Surface Methodology
3.2. Structural Characterization by Mass Spectrometry
3.3. Sensory Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Prevention and Control of Noncommunicable Diseases in the European Region: A Progress Report; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Bellisle, F.; Drewnowski, A. Intense sweeteners, energy intake and the control of body weight. Eur. J. Clin. Nutr. 2007, 61, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Rogers, P.J.; Hogenkamp, P.S.; de Graaf, C.; Higgs, S.; Lluch, A.; Ness, A.R.; Penfold, C.; Perry, R.; Putz, P.; Yeomans, M.R.; et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes. 2016, 40, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef]
- O’Mullane, M.; Fields, B.; Stanley, G. Food Additives: Sweeteners; Encyclopedia of Food Safety; Academic Press: Waltham, MA, USA, 2014; pp. 477–484. ISBN 978-0-12-378613-5. [Google Scholar]
- Prakash, I.; Campbell, M.; Miguel, R.I.S.; Chaturvedula, V.S.P. Synthesis and sensory evaluation of ent-kaurane diterpene glycosides. Molecules 2012, 17, 8908–8916. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the safety of steviol glycosides for the proposed uses as a food additive. EFSA J. 2010, 8, 1537. [Google Scholar] [CrossRef]
- Perrier, J.D.; Mihalov, J.J.; Carlson, S.J. FDA regulatory approach to steviol glycosides. Food Chem. Toxicol. 2018, 122, 132–142. [Google Scholar] [CrossRef]
- Kurek, J.M.; Krejpcio, Z. The functional and health-promoting properties of Stevia rebaudiana Bertoni and its glycosides with special focus on the antidiabetic potential—A review. J. Funct. Foods 2019, 61, 103465. [Google Scholar] [CrossRef]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.I. Sweeteners from plants—With emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Gerwig, G.J.; te Poele, E.M.; Dijkhuizen, L.; Kamerling, J.P. Stevia Glycosides: Chemical and Enzymatic Modifications of Their Carbohydrate Moieties to Improve the Sweet-Tasting Quality. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 73, pp. 1–72. [Google Scholar]
- Amiri, A.; Mohamad, R.; Rahim, R.A.; Illias, R.M.; Namvar, F.; Tan, J.S.; Abbasiliasi, S. Cyclodextrin glycosyltransferase biosynthesis improvement by recombinant Lactococcus lactis NZ:NSP:CGT: Medium formulation and culture condition optimization. Biotechnol. Biotechnol. Equip. 2015, 29, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Shwetha, V.; More, S.S.; Uday, M. Screening, optimization and production of a novel β-cyclodextrinase by Bacillus flexus MSBC 2. Curr. Trends Biotechnol. Pharm. 2017, 11, 136–143. [Google Scholar]
- Wang, F.; Du, G.; Li, Y.; Chen, J. Optimization of cultivation conditions for the production of γ-cyclodextrin glucanotransferase by Bacillus macorous. Food Biotechnol. 2004, 18, 251–264. [Google Scholar] [CrossRef]
- Pól, J.; Varaďová Ostrá, E.; Karásek, P.; Roth, M.; Benešová, K.; Kotlaříková, P.; Čáslavský, J. Comparison of two different solvents employed for pressurised fluid extraction of stevioside from Stevia rebaudiana: Methanol versus water. Anal. Bioanal. Chem. 2007, 388, 1847–1857. [Google Scholar] [CrossRef]
- Espinoza, M.I.; Vincken, J.-P.; Sanders, M.; Castro, C.; Stieger, M.; Agosin, E. Identification, Quantification, and Sensory Characterization of Steviol Glycosides from Differently Processed Stevia rebaudiana Commercial Extracts. J. Agric. Food Chem. 2014, 62, 11797–11804. [Google Scholar] [CrossRef]
- Prakash, I.; Chaturvedula, V. Structures of Some Novel α-Glucosyl Diterpene Glycosides from the Glycosylation of Steviol Glycosides. Molecules 2014, 19, 20280–20294. [Google Scholar] [CrossRef] [Green Version]
- Lindley, M.G. Natural High-Potency Sweeteners. In Sweeteners and Sugar Alternatives in Food Technology; Wiley Online Books; Wiley-Blackwell: Oxford, UK, 2012; pp. 185–212. ISBN 9781118373941. [Google Scholar]
- Singla, R.; Jaitak, V. Synthesis of rebaudioside A from stevioside and their interaction model with hTAS2R4 bitter taste receptor. Phytochemistry 2016, 125, 106–111. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Li, Y.; Li, Y.; Yan, M.; Chen, K.; Hao, N.; Xu, L. Efficient enzymatic production of rebaudioside A from stevioside. Biosci. Biotechnol. Biochem. 2016, 80, 67–73. [Google Scholar] [CrossRef]
- Kochikyan, V.T.; Markosyan, A.A.; Abelyan, L.A.; Balayan, A.M.; Abelyan, V.A. Combined enzymatic modification of stevioside and rebaudioside A. Appl. Biochem. Microbiol. 2006, 42, 31–37. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of Novel Steviol Glycosides from Leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Devlamynck, T.; te Poele, E.M.; Quataert, K.; Gerwig, G.J.; Van de Walle, D.; Dewettinck, K.; Kamerling, J.P.; Soetaert, W.; Dijkhuizen, L. Trans-α-glucosylation of stevioside by the mutant glucansucrase enzyme Gtf180-ΔN-Q1140E improves its taste profile. Food Chem. 2019, 272, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Jaitak, V.; Kaul, V.K.; Bandna; Kumar, N.; Singh, B.; Savergave, L.S.; Jogdand, V.V.; Nene, S. Simple and efficient enzymatic transglycosylation of stevioside by β-cyclodextrin glucanotransferase from Bacillus firmus. Biotechnol. Lett. 2009, 31, 1415. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Miyata, T.; Nakayasu, N.; Mizutani, K.; Kasai, R.; Tanaka, O.; Tanaka, O. Enzymic transglucosylation products of stevioside: Separation and sweetness-evaluation. Agric. Biol. Chem. 1989, 53, 1603–1607. [Google Scholar]
- Abelyan, V.A.; Balayan, A.M.; Ghochikyan, V.T.; Markosyan, A.A. Transglycosylation of stevioside by cyclodextrin glucanotransferases of various groups of microorganisms. Appl. Biochem. Microbiol. 2004, 40, 129–134. [Google Scholar] [CrossRef]
- Uitdehaag, J.C.M.; van Alebeek, G.-J.W.M.; van der Veen, B.A.; Dijkhuizen, L.; Dijkstra, B.W. Structures of Maltohexaose and Maltoheptaose Bound at the Donor Sites of Cyclodextrin Glycosyltransferase Give Insight into the Mechanisms of Transglycosylation Activity and Cyclodextrin Size Specificity. Biochemistry 2000, 39, 7772–7780. [Google Scholar] [CrossRef] [Green Version]
- Younes, M.; Aquilina, G.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; Mennes, W.; et al. Safety of use of Monk fruit extract as a food additive in different food categories. EFSA J. 2019, 17, e05921. [Google Scholar]
- Purkayastha, S.; Markosyan, A.; Prakash, I.; Bhusari, S.; Pugh, G.; Lynch, B.; Roberts, A. Steviol glycosides in purified stevia leaf extract sharing the same metabolic fate. Regul. Toxicol. Pharmacol. 2016, 77, 125–133. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Di Michele, L.; Laino, G.; Vajro, P. Obesity and Obesity Related Diseases, Sugar Consumption and Bad Oral Health: A Fatal Epidemic Mixtures: The Pediatric and Odontologist Point of View. Transl. Med. @ UniSa 2017, 16, 11–16. [Google Scholar]





| Factors | Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Synthesized Glucosylated SVglys (mg/mL) | Synthesized Glucosylated RebA (mg/mL) | |||||
| Run | Maltodextrin (mg/mL) | Unmodified SVglys or RebA (mg/mL) | Enzyme Activity (U/g acceptor) | Temp (°C) | Time (h) | pH | CGTase Geobacillus sp. | CGTase Paenibacillus macerans | CGTase Thermoanaerobacter sp. | CGTase Geobacillus sp. | CGTase Paenibacillus macerans | CGTase Thermoanaerobacter sp. |
| 1 | 5 | 50 | 5 | 70 | 6 | 5 | 0.83 | 0.66 | 0.80 | 1.85 | 0.06 | 2.49 |
| 2 | 5 | 5 | 5 | 70 | 1 | 7 | 0.92 | 0.22 | 1.50 | 2.69 | 0.00 | 2.04 |
| 3 | 5 | 50 | 25 | 50 | 1 | 5 | 1.07 | 0.55 | 1.25 | 2.78 | 0.21 | 2.92 |
| 4 | 5 | 50 | 5 | 50 | 6 | 7 | 9.65 | 7.85 | 9.83 | 28.51 | 4.07 | 28.49 |
| 5 | 50 | 5 | 5 | 50 | 6 | 5 | 0.84 | 0.59 | 1.13 | 1.94 | 0.47 | 2.28 |
| 6 | 5 | 50 | 25 | 70 | 1 | 7 | 3.10 | 1.21 | 4.26 | 8.97 | 1.83 | 12.16 |
| 7 | 50 | 50 | 25 | 70 | 6 | 7 | 0.98 | 0.77 | 0.76 | 2.23 | 0.48 | 2.04 |
| 8 | 50 | 5 | 5 | 70 | 6 | 7 | 3.34 | 8.70 | 4.49 | 3.01 | 0.00 | 12.16 |
| 9 | 50 | 50 | 25 | 50 | 6 | 5 | 6.19 | 4.01 | 9.65 | 26.04 | 3.71 | 25.79 |
| 10 | 50 | 50 | 5 | 50 | 1 | 7 | 8.70 | 2.12 | 10.86 | 22.24 | 0.41 | 24.98 |
| 11 | 5 | 5 | 25 | 50 | 6 | 7 | 2.07 | 2.23 | 4.02 | 6.81 | 1.87 | 10.32 |
| 12 | 50 | 5 | 25 | 70 | 1 | 5 | 1.55 | 0.73 | 0.98 | 2.68 | 0.00 | 3.16 |
| 13 | 5 | 5 | 5 | 50 | 1 | 5 | 3.96 | 2.96 | 4.47 | 11.20 | 1.83 | 11.24 |
| 14 | 50 | 5 | 25 | 50 | 1 | 7 | 1.10 | 0.17 | 1.02 | 1.33 | 0.00 | 2.92 |
| 15 | 5 | 5 | 25 | 70 | 6 | 5 | 11.33 | 3.73 | 11.06 | 27.71 | 1.54 | 26.45 |
| 16 | 50 | 50 | 5 | 70 | 1 | 5 | 1.32 | 1.09 | 1.48 | 2.75 | 0.00 | 3.12 |
| Response | Theoretical Result a | Experimental Result bc | Confidence Interval d | |
|---|---|---|---|---|
| (-) | ( + ) | |||
| Synthesized glucosylated SVglys (mg/mL) | 15.8 | 17.3 ± 1.0 (6.0%) | 12.5 | 19.1 |
| Synthesized glucosylated RebA (mg/mL) | 26.2 | 26.1 ± 2.4 (9.3%) | 22.1 | 30.3 |
| Sucrose Equivalent (%) | Potency | |
|---|---|---|
| SVglys | 4.5 ± 2.8 * | 140 ± 86 |
| Modified SVglys | 4.5 ± 2.5 | 140 ± 78 |
| RebA | 5.3 ± 2.7 | 223 ± 112 |
| Modified RebA | 4.7 ± 2.5 | 194 ± 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Labrador, A.; Azcarate, S.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Galindo-Iranzo, P.; Kolida, S.; Methven, L.; Rastall, R.A.; Moreno, F.J.; Hernandez-Hernandez, O. Transglycosylation of Steviol Glycosides and Rebaudioside A: Synthesis Optimization, Structural Analysis and Sensory Profiles. Foods 2020, 9, 1753. https://doi.org/10.3390/foods9121753
Muñoz-Labrador A, Azcarate S, Lebrón-Aguilar R, Quintanilla-López JE, Galindo-Iranzo P, Kolida S, Methven L, Rastall RA, Moreno FJ, Hernandez-Hernandez O. Transglycosylation of Steviol Glycosides and Rebaudioside A: Synthesis Optimization, Structural Analysis and Sensory Profiles. Foods. 2020; 9(12):1753. https://doi.org/10.3390/foods9121753
Chicago/Turabian StyleMuñoz-Labrador, Ana, Silvana Azcarate, Rosa Lebrón-Aguilar, Jesús E. Quintanilla-López, Plácido Galindo-Iranzo, Sofia Kolida, Lisa Methven, Robert A. Rastall, F. Javier Moreno, and Oswaldo Hernandez-Hernandez. 2020. "Transglycosylation of Steviol Glycosides and Rebaudioside A: Synthesis Optimization, Structural Analysis and Sensory Profiles" Foods 9, no. 12: 1753. https://doi.org/10.3390/foods9121753