Spontaneously Fermented Fruiting Bodies of Agaricus bisporus as a Valuable Source of New Isolates of Lactic Acid Bacteria with Functional Potential
Abstract
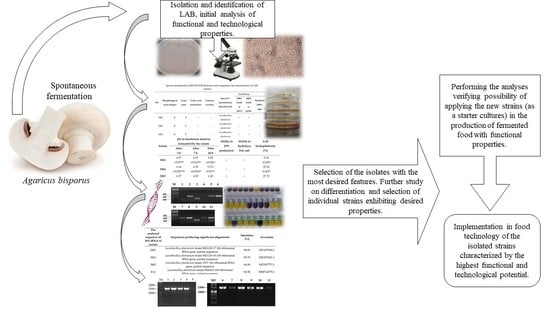
:1. Introduction
2. Materials and Methods
2.1. Spontaneous Fermentation of Fungal Raw Material
2.2. Isolation and Selection of LAB Isolates
2.3. Preliminary Identification of Isolates Using the MALDI-TOF Biotyper
2.4. Comparison of the Selected Technological Functional Properties of Selected Autochthonous LAB
2.4.1. Ability of Isolates to Grow on Medium with an Alternative Carbon Source
2.4.2. Dynamics of Acidification of the Mushroom Material
2.4.3. Detection of Isolates Able to Produce Exopolysaccharides
2.4.4. Detection of Acid Tolerance in LAB Isolates
2.4.5. Hydrophobicity of Bacterial Cells
2.4.6. Ability to Hydrolyze Bile Salt
2.5. Further Molecular and Biochemical Analysis of Selected LAB Isolates
2.5.1. 16S rRNA Gene Sequencing Analysis
2.5.2. Species-Specific Multiplex-PCR
2.6. Statistical Analysis
3. Results
3.1. Identification of Autochthonous LAB in Spontaneously Fermented A. bisporus
3.2. Comparison of the Selected Technological Functional Properties of Selected Autochthonous LAB
4. Discussion
4.1. Identification and Screening of Autochthonous LAB in Spontaneously Fermented A. bisporus
4.2. Comparison of the Selected Technological and Functional Properties of the Chosen Autochthonous LAB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 3, 197–212. [Google Scholar] [CrossRef]
- FAO/WHO. Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation; FAO: Rome, Italy, 2002; pp. 1–59. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sáez, G.D.; Hébert, E.M.; Saavedra, L.; Zárate, G. Molecular identification and technological characterization of lactic acid bacteria isolated from fermented kidney beans flours (Phaseolus vulgaris L. and P. coccineus) in northwestern. Food Res. Int. 2017, 102, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Ryś, E.; Skrzypczak, K.; Sławińska, A.; Radzki, W.; Gustaw, W. Lactic acid fermentation of edible mushrooms: Tradition, technology, current state of research: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 265–669. [Google Scholar] [CrossRef] [Green Version]
- Finimundy, T.C.; Gambato, G.; Fontana, R.; Camassola, M.; Salvador, M.; Moura, S.; Hess, J.; Henriques, J.A.P.; Dillon, A.J.P.; Roesch-Ely, M. Aqueous extracts of Lentinula edodes and Pleurotus sajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutr. Res. 2013, 33, 76–84. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Kim, Y.I.; Lee, Y.H.; Kim, K.H.; Oh, Y.K.; Moon, Y.H.; Kwak, W.S. Effects of supplementing microbially-fermented spent mushroom substrates on growth performance and carcass characteristics of Hanwoo steers (a field study). Asian Australas J. Anim. Sci. 2012, 25, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Baek, Y.C.; Kim, M.S.; Reddy, K.E.; Oh, Y.K.; Jung, Y.H.; Yeo, J.M.; Choi, H. Rumen fermentation and digestibility of spent mushroom (Pleurotus ostreatus) substrate inoculated with Lactobacillus brevis for Hanwoo steers. Rev. Colomb. Cienc. Pecu. 2017, 30, 267–277. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yanagida, F.; Hsu, J.-S. Isolation and characterization of lactic acid bacteria from dochi (fermented black beans), a traditional fermented food in Taiwan. Lett. Appl. Microbiol. 2006, 43, 229–235. [Google Scholar] [CrossRef]
- Waśko, A.; Szwajgier, D.; Polak-Berecka, M. The role of ferulic acid esterase in the growth of Lactobacillus helveticus in the presence of phenolic acids and their derivatives. Eur. Food Res. Technol. 2014, 238, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Coeuret, V.; Dubernet, S.; Bernardeau, M.; Guegun, M.; Vernouxj, P. Isolation, characterization and identification of lactobacilli focusing mainly on cheeses and other dairy products. Le Lait 2003, 83, 269–306. [Google Scholar] [CrossRef]
- Tajabadi, N.; Mardan, M.; Saari, N.; Mustafa, S.; Bahreini, R.; Manap, M.Y. Identification of Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus fermentum from honey stomach of honeybee. Braz. J. Microbiol. 2014, 44, 717–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustaw, K.; Michalak, M.; Polak-Berecka, M.; Waśko, A. Isolation and characterization of a new fructophilic Lactobacillus plantarum FPL strain from honeydew. Ann. Microbiol. 2018, 68, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Jabłońska-Ryś, E.; Stachniuk, A. High-performance liquid chromatography determination of Free sugars and mannitol in mushrooms using corona charged aerosol detection. Food Anal. Methods 2020, in press. [Google Scholar] [CrossRef]
- Manini, F.; Casiraghi, M.C.; Poutanen, K.; Brasca, M.; Erba, D.; Plumed-Ferrer, C. Characterization of lactic acid bacteria isolated from wheat bran sourdough. LWT Food Sci. Technol. 2016, 66, 275–283. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; De Los Reyes-Gavilan, C.G. Invited review: Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, T.; Balcázar, J.L.; García, Y.; Halaihel, N.; Vendrell, D.; De Blas, I.; Merrifield, D.L.; Ruiz-Zarzuela, I. Identification and characterization of lactic acid bacteria isolated from rainbow trout, Oncorhynchus mykiss (Walbaum), with inhibitory activity against Lactococcus garvieae. J. Fish Dis. 2011, 34, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.C.; Rodrigues, M.R.; Winkelströter, L.K.; Nomizo, A.; de Martinis, E.C. In vitro evaluation of the probiotic potential of bacteriocin producer Lactobacillus sakei 1. J. Food Prot. 2012, 75, 83–89. [Google Scholar] [CrossRef]
- Franz, C.M.; Specht, I.; Haberer, P.; Holzapfel, W.H. Bile salt hydrolase activity of enterococci isolated from food: Screening and quantitative determination. J. Food Prot. 2001, 64, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA Gene Sequence Analysis and Multiplex PCR Assay with recA Gene-Derived Primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://lactotax.embl.de/wuyts/lactotax/ (accessed on 20 September 2020).
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanić, K.; Matošić, S. Antimicrobial activity—The most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Beganović, J.; Kos, B.; Leboš Pavunc, A.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Axelsson, L.; Naterstad, K.; Elsser, D.; Budde, B.B. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. Food Microbiol. 2005, 105, 419–431. [Google Scholar] [CrossRef]
- Beganović, J.; Guillot, A.; van de Guchte, M.; Jouan, A.; Gitton, C.; Loux, V.; Roy, K.; Huet, S.; Monod, H.; Monnet, V. Characterization of the insoluble proteome of Lactococcus lactis by SDS-PAGE LC–MS/MS leads to the identification of new markers of adaptation of the bacteria to the mouse digestive tract. J. Proteome Res. 2010, 9, 677–688. [Google Scholar] [CrossRef]
- Delgado, S.; Flórez, A.B.; Mayo, B. Antibiotic susceptibility of Lactobacillus and Bifidobacterium species from the human gastrointestinal tract. Curr. Microbiol. 2005, 50, 202–207. [Google Scholar] [CrossRef]
- Breidt, F.J. A genomic study of Leuconostoc mesenteroides and the molecular ecology of sauerkraut fermentations. J. Food Sci. 2008, 69, FMS30–FMS33. [Google Scholar] [CrossRef]
- Palys, T.; Nakamura, L.K.; Cohan, F.M. Discovery and classification of ecological diversity in the bacterial world: The role of DNA sequence data. Int. J. Syst. Bacteriol. 1997, 47, 1145–1156. [Google Scholar] [CrossRef]
- Batista, N.N.; Ramos, C.L.; de Figueiredo Vilela, L.; Dias, D.R.; Schwan, R.F. Fermentation of yam (Dioscorea spp. L.) by indigenous phytase-producing lactic acid bacteria strains. Braz. J. Microbiol. 2019, 50, 507–514. [Google Scholar] [CrossRef]
- Johanningsmeier, S.D.; McFeeters, R.F.; Fleming, H.P.; Thompson, R.L. Effects of Leuconostoc mesenteroides starter culture on fermentation of cabbage with reduced salt concentrations. J. Food Sci. 2007, 72, 166–172. [Google Scholar] [CrossRef]
- Plengvidhya, V.; Breid, F.J.; Lu, Z.; Fleming, H.P. DNA fingerprinting of lactic acid bacteria in sauerkraut fermentation. Appl. Environ. Microbiol. 2007, 73, 7697–7702. [Google Scholar] [CrossRef] [Green Version]
- Khaskheli, S.G.; Zheng, W.; Sheikh, S.A.; Khaskheli, A.A.; Liu, Y.; Wang, Y.F.; Huang, W. Effect of processing techniques on the quality and acceptability of Auricularia auricula mushroom pickle. J. Food Nutr. Res. 2015, 3, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Buntin, N.; Chanthachum, S.; Hongpattarakere, T. Screening of lactic acid bacteria from gastrointestinal tracts of marine fish for their potential use as probiotics. SJST 2008, 30, 141–148. [Google Scholar]
- Menconi, A.; Kallapura, G.; Latorre, J.D.; Morganm, J.; Pumford, N.R.; Hargis, B.M.; Tellez, G. Identification and characterization of lactic acid bacteria in a commercial probiotic culture. BMFH 2014, 33, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Huang, S.; Zhang, Y.; Li, H.; Zhou, S. Characterization of extracellular polymeric substances produced during nitrate removal by a thermophilic bacterium Chelatococcus daeguensis TAD1 in batch cultures. RSC Adv. 2017, 7, 44265–44271. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.F.; Li, Q.; Tan, L.R.; Sun, X.F.; Song, C.; Wang, S.G. Extracellular polymeric substances protect Escherichia coli from organic solvents. RSC Adv. 2016, 6, 59438–59444. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Varinauskaite, I.; Pileckaite, G.; Paskeviciute, L.; Rutkauskaite, G.; Kanaporis, T.; et al. Plants and lactic acid bacteria combination for new antimicrobial and antioxidant properties product development in a sustainable manner. Foods 2020, 9, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosseva, M.R. Management and Processing of Food Wastes. In Comprehensive Biotechnology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 6, pp. 558–593. [Google Scholar]
| Isolate ID * | Morphological Form (Shape) | Gram Stain | Lactic Acid Production | Catalase Reaction | Species ** Identified by MALDI-TOF | Growth in | ||
|---|---|---|---|---|---|---|---|---|
| MRS Broth at pH = 3 | MRS Broth at pH = 4 | MSodified MRS Agar | ||||||
| EK1, E56, E57, E58, E59, E60, E61 | R | P | + | - | Lactiplantibacillus plantarum | + | + | _ |
| EK2, E14 | R | P | + | - | Lactiplantibacillus plantarum | - | + | + |
| EK3, EK5, EK6, EK10, EK11, EK12, EK13, EK15, EK51, EK55, E62 | R | P | + | - | Lactiplantibacillus plantarum | + | + | + |
| EK7, EK8, EK9, E21, E22, E26, E65, E66, E67, E100, E101, E102, E103, E104, E120, E121, E122, E123, E124, E73B, E125, E126, E127, E128 | C | P | + | - | Lactococcus lactis | - | - | - |
| E64, E68, E74B, E75B | C | P | + | - | Lactococcus lactis | - | - | + |
| E79A | C | P | + | - | Lactococcus lactis | + | + | - |
| E23, E24A, E25, E96C, E76B | C | P | + | - | Leuconostoc mesenteroides | - | - | - |
| E88B, E72C | C | P | + | - | Leuconostoc mesenteroides | + | + | + |
| E71C, E76C, E79C, E80C, E81B, E82B, E83B, E84A, E96C, E71A, E73A, E72A, E74A, E75A, E76A, E78A, E71B, E84B, E93C, E94C, E95C, E81A, E83A, E82A, E80A, E86B, E73C, E78B, E77B, E77A, E79B, E80B, E89B, E74C, E75C, E78C, E81C, E82C, E83C, E84C, E72B, E85C, E86C, E87C, E88C, E89C, E90C, E91C, E92C, E87B, E85B | C | P | + | - | Leuconostoc mesenteroides | - | - | + |
| E53, E54 | R | P | + | - | Lactiplantibacillus paraplantarum | - | - | + |
| EK4 | R | P | + | - | Lactiplantibacillus paraplantarum | + | + | + |
| Isolate | pH of Mushroom Material Fermented by the Isolate | Ability to EPS Production | Ability to Hydrolyze Bile Salt | Cell Hydrophobicity [%] | ||
|---|---|---|---|---|---|---|
| After 3 h | After 7 h | After 24 h | ||||
| EK3 | 6.27 ± 0.07 abB | 6.33 ± 0.012 efB | 6.02 ± 0.026 jA | + | + | 0.34 ± 0.292 a |
| EK4 | 6.36 ± 0.052 cdefB | 6.35 ± 0.010 fgB | 5.17 ± 0.015 bA | + | + | 25.34 ± 0.407 e |
| EK5 | 6.37 ± 0.025 cdefC | 6.29 ± 0.010 bcdB | 6.20 ± 0.015 kA | + | + | 27.33 ± 1.516 f |
| EK6 | 6.30 ± 0.021 abcdA | 6.39 ± 0.006 hA | 5.70 ± 0.010 eA | + | + | 0.37 ± 0.112 a |
| EK10 | 6.42 ± 0.053 aC | 6.23 ± 0.006 cdeB | 5.69 ± 0.010 gA | - | + | n.d. |
| EK11 | 6.54 ± 0.089 abcB | 6.19 ± 0.006 bcdB | 5.71 ± 0.010 hA | - | - | n.d. |
| EK12 | 6.39 ± 0.055 efB | 6.34 ± 0.010 efgB | 5.40 ± 0.015 cA | + | + | 29.59 ± 0.700 g |
| EK13 | 6.39 ± 0.026 defB | 6.37 ± 0.010 ghB | 6.01 ± 0.021 jA | + | + | 0.11 ± 0.113 a |
| EK15 | 6.48 ± 0.071 bcdeC | 6.21 ± 0.006 abB | 5.70 ± 0.010 fA | + | + | 4.66 ± 0.918 b |
| EK51 | 6.34 ± 0.006 bcdefC | 6.28 ± 0.010 abcB | 5.79 ± 0.010 fA | + | + | 8.83 ± 0.104 c |
| EK55 | 6.35 ± 0.006 bcdefB | 6.32 ± 0.015 efB | 5.06 ± 0.021 aA | + | + | 28.75 ± 0.551 fg |
| E62 | 6.31 ± 0.012 abcdA | 6.29 ± 0.006 bcB | 5.94 ± 0.010 iA | + | + | 1.52 ± 0.435 a |
| E24A | 6.34 ± 0.017 bcdeB | 6.27 ± 0.015 bcdB | 5.77 ± 0.006 fgA | + | - | n.d. |
| E72C | 6.34 ± 0.006 bcdeB | 6.32 ± 0.010 defB | 5.54 ± 0.010 dA | + | - | 16.63 ± 2.217 d |
| E79B | 6.29 ± 0.017 abcB | 6.27 ± 0.006 abB | 5.67 ± 0.010 eA | - | - | n.d. |
| E88B | 6.35 ± 0.035 bcdefC | 6.25 ± 0.006 aB | 5.68 ± 0.010 eA | + | - | 14.68 ± 0.151 d |
| Control | 6.43 ± 0.017 fB | 6.42 ± 0.010 iAB | 6.4 ± 0.010 lA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypczak, K.; Gustaw, K.; Jabłońska-Ryś, E.; Sławińska, A.; Gustaw, W.; Winiarczyk, S. Spontaneously Fermented Fruiting Bodies of Agaricus bisporus as a Valuable Source of New Isolates of Lactic Acid Bacteria with Functional Potential. Foods 2020, 9, 1631. https://doi.org/10.3390/foods9111631
Skrzypczak K, Gustaw K, Jabłońska-Ryś E, Sławińska A, Gustaw W, Winiarczyk S. Spontaneously Fermented Fruiting Bodies of Agaricus bisporus as a Valuable Source of New Isolates of Lactic Acid Bacteria with Functional Potential. Foods. 2020; 9(11):1631. https://doi.org/10.3390/foods9111631
Chicago/Turabian StyleSkrzypczak, Katarzyna, Klaudia Gustaw, Ewa Jabłońska-Ryś, Aneta Sławińska, Waldemar Gustaw, and Stanisław Winiarczyk. 2020. "Spontaneously Fermented Fruiting Bodies of Agaricus bisporus as a Valuable Source of New Isolates of Lactic Acid Bacteria with Functional Potential" Foods 9, no. 11: 1631. https://doi.org/10.3390/foods9111631