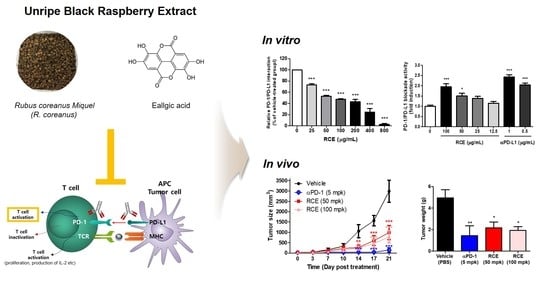
Unripe Black Raspberry (Rubus coreanus Miquel) Extract and Its Constitute, Ellagic Acid Induces T Cell Activation and Antitumor Immunity by Blocking PD-1/PD-L1 Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RCE
2.3. PD-1/PD-L1 Competitive ELISA
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Cellular PD-1/PD-L1 Blockade Assay
2.7. Cytokine Measurement
2.8. Animals with Experiments
2.9. In Vivo Evaluation Using hPD-L1 MC38 Tumor Model in Humanized PD-1 Mice
2.10. High-Performance Liquid Chromatography (HPLC) Analysis
2.11. Preparation of Ellagic Acid–Sepharose 4B Beads
2.12. In Vitro Pull-Down Assays
2.13. Western Blot
2.14. Statistical Analysis
3. Results
3.1. Effects of RCE on the PD-1/PD-L1 Protein Interaction and T Cell Function In Vitro
3.2. Anti-Tumor Effect of RCE on Tumor Growth of MC38 Expressing hPD-L1 in Humanized PD-1 Mice
3.3. Identification of the Main Compound of RCE by HPLC Analysis
3.4. Potent Biological Effects of the Main Compound of RCE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AOM | Azoxymethane |
| bw | Body weight |
| Caco-2 | Colon adenocarcinoma isolated from a primary colonic tumor |
| CRC | Colorectal cancer |
| EC50 | A concentration of a drug that gives half-maximal response |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| TCR | T-cell receptor |
| NFAT | Nuclear factor of activated T cells |
| mpk | mg/kg |
| IC50 | Half maximal inhibitory concentration |
| IL | Interleukin |
| i.p. | Intraperitoneal injection |
| i.g. | Intragastric administration |
| ICI | Immune checkpoint inhibitor |
| PPI | Protein-protein interaction |
| s.c. | Subcutaneously |
| N/D | Not determined or not reported |
| wk | Week |
References
- Kaufman, H.L.; Atkins, M.B.; Subedi, P.; Wu, J.; Chambers, J.; Joseph Mattingly, T., II; Campbell, J.D.; Allen, J.; Ferris, A.E.; Schilsky, R.L.; et al. The promise of Immuno-oncology: Implications for defining the value of cancer treatment. J. Immunother. Cancer 2019, 7, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalniak, L.; Zak, K.M.; Guzik, K.; Magiera, K.; Musielak, B.; Pachota, M.; Szelazek, B.; Kocik, J.; Grudnik, P.; Tomala, M.; et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 2017, 8, 72167–72181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calik, I.; Calik, M.; Turken, G.; Ozercan, I.H.; Dagli, A.F.; Artas, G.; Sarikaya, B. Intratumoral Cytotoxic T-Lymphocyte Density and PD-L1 Expression Are Prognostic Biomarkers for Patients with Colorectal Cancer. Medicina 2019, 55, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, A.; Ahmed, M.; Okoye, I.; Arutyunova, E.; Babu, D.; Turnbull, W.L.; Kundu, J.K.; Shields, J.; Agopsowicz, K.C.; Xu, L.; et al. Comprehensive in vitro characterization of PD-L1 small molecule inhibitors. Sci. Rep. 2019, 9, 12392. [Google Scholar] [CrossRef] [PubMed]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2020. [Google Scholar] [CrossRef]
- Shimozaki, K.; Sukawa, Y.; Beppu, N.; Kurihara, I.; Suzuki, S.; Mizuno, R.; Funakoshi, T.; Ikemura, S.; Tsugaru, K.; Togasaki, K.; et al. Multiple Immune-Related Adverse Events and Anti-Tumor Efficacy: Real-World Data on Various Solid Tumors. Cancer Manag. Res. 2020, 12, 4585–4593. [Google Scholar] [CrossRef]
- Yu, G.; Luo, Z.; Wang, W.; Li, Y.; Zhou, Y.; Shi, Y. Rubus chingii Hu: A Review of the Phytochemistry and Pharmacology. Front. Pharmacol. 2019, 10, 799. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, E.; Lee, J.E.; Auh, J.H.; Choi, H.K.; Lee, J.; Cho, S.; Kim, J.H. Effects of a Rubus coreanus Miquel supplement on plasma antioxidant capacity in healthy Korean men. Nutr. Res. Pract. 2011, 5, 429–434. [Google Scholar] [CrossRef]
- Lim, J.W.; Hwang, H.J.; Shin, C.S. Polyphenol compounds and anti-inflammatory activities of Korean black raspberry (Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J. Agric. Food Chem. 2012, 60, 5121–5127. [Google Scholar] [CrossRef]
- Wang, J.; Fei, K.; Jing, H.; Wu, Z.; Wu, W.; Zhou, S.; Ni, H.; Chen, B.; Xiong, Y.; Liu, Y.; et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs 2019, 11, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Choi, H.S.; Cho, S.G.; Shin, Y.C.; Ko, S.G. Rubus coreanus Miquel extract causes apoptosis of doxorubicin-resistant NCI/ADR-RES ovarian cancer cells via JNK phosphorylation. Mol. Med. Rep. 2016, 13, 4065–4072. [Google Scholar] [CrossRef]
- Li, W.; Kim, T.I.; Kim, J.H.; Chung, H.S. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules 2019, 24, 4062. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, Y.S.; Choi, J.G.; Li, W.; Lee, E.J.; Park, J.W.; Song, J.; Chung, H.S. Kaempferol and Its Glycoside, Kaempferol 7-O-Rhamnoside, Inhibit PD-1/PD-L1 Interaction In Vitro. Int. J. Mol. Sci. 2020, 21, 3239. [Google Scholar] [CrossRef]
- Park, H.E.; Kang, K.W.; Kim, B.S.; Lee, S.M.; Lee, W.K. Immunomodulatory Potential of Weissella cibaria in Aged C57BL/6J Mice. J. Microbiol. Biotechnol. 2017, 27, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCdelta and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [Green Version]
- Zhai, W.; Zhou, X.; Du, J.; Gao, Y. In vitro assay for the development of small molecule inhibitors targeting PD-1/PD-L1. Methods Enzymol. 2019, 629, 361–381. [Google Scholar] [CrossRef]
- Basu, S.; Yang, J.; Xu, B.; Magiera-Mularz, K.; Skalniak, L.; Musielak, B.; Kholodovych, V.; Holak, T.A.; Hu, L. Design, Synthesis, Evaluation, and Structural Studies of C2-Symmetric Small Molecule Inhibitors of Programmed Cell Death-1/Programmed Death-Ligand 1 Protein-Protein Interaction. J. Med. Chem. 2019, 62, 7250–7263. [Google Scholar] [CrossRef]
- Li, Q.X.; Feuer, G.; Ouyang, X.; An, X. Experimental animal modeling for immuno-oncology. Pharmacol. Ther. 2017, 173, 34–46. [Google Scholar] [CrossRef]
- Olson, B.; Li, Y.; Lin, Y.; Liu, E.T.; Patnaik, A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018, 8, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Peng, D.; Guo, H.; Ben, Y.; Zuo, X.; Wu, F.; Yang, X.; Teng, F.; Li, Z.; Qian, X.; et al. A human programmed death-ligand 1-expressing mouse tumor model for evaluating the therapeutic efficacy of anti-human PD-L1 antibodies. Sci. Rep. 2017, 7, 42687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.Y.; Wang, S.Q.; Liu, K.H.; Zhu, B.; Zhang, Q.Y.; Qin, L.P.; Wu, J.J. Rubus chingii Hu: An overview of botany, traditional uses, phytochemistry, and pharmacology. Chin. J. Nat. Med. 2020, 18, 401–416. [Google Scholar] [CrossRef]
- Nunez-Sanchez, M.A.; Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Garcia-Villalba, R.; Selma, M.V.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Dietary phenolics against colorectal cancer—From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Wang, L.S.; Kuo, C.T.; Cho, S.J.; Seguin, C.; Siddiqui, J.; Stoner, K.; Weng, Y.I.; Huang, T.H.; Tichelaar, J.; Yearsley, M.; et al. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr. Cancer 2013, 65, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2’-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef]
- Bi, X.; Fang, W.; Wang, L.S.; Stoner, G.D.; Yang, W. Black raspberries inhibit intestinal tumorigenesis in apc1638+/- and Muc2-/- mouse models of colorectal cancer. Cancer Prev. Res. 2010, 3, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.D.; Deng, Y.R.; Tian, Z.; Lian, Z.X. Traditional Chinese medicine and immune regulation. Clin. Rev. Allergy Immunol. 2013, 44, 229–241. [Google Scholar] [CrossRef]
- Lu, X.; Wu, X.; Jing, L.; Tao, L.; Zhang, Y.; Huang, R.; Zhang, G.; Ren, J. Network Pharmacology Analysis and Experiments Validation of the Inhibitory Effect of JianPi Fu Recipe on Colorectal Cancer LoVo Cells Metastasis and Growth. Evid. Based Complement. Altern Med. 2020, 2020, 4517483. [Google Scholar] [CrossRef]
- He, Y.; Jin, S.; Ma, Z.; Zhao, J.; Yang, Q.; Zhang, Q.; Zhao, Y.; Yao, B. The antioxidant compounds isolated from the fruits of chinese wild raspberry Rubus Chingii Hu. Nat. Prod. Res. 2020, 34, 872–875. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef]
- Noh, K.T.; Cha, G.S.; Kim, H.C.; Lee, J.H.; Ahn, S.C.; Kim, D.K.; Park, Y.M. Ellagic acid modulates LPS-induced maturation of dendritic cells through the regulation of JNK activity. J. Med. Food 2014, 17, 996–1002. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.K.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Bo, W.; Zhou, Y.; Dang, S.; Wei, J.; Li, X.; Liu, M. Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. Int. J. Oncol. 2017, 50, 613–621. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, Y.S.; Kim, T.I.; Li, W.; Mun, J.-G.; Jeon, H.D.; Kee, J.-Y.; Choi, J.-G.; Chung, H.-S. Unripe Black Raspberry (Rubus coreanus Miquel) Extract and Its Constitute, Ellagic Acid Induces T Cell Activation and Antitumor Immunity by Blocking PD-1/PD-L1 Interaction. Foods 2020, 9, 1590. https://doi.org/10.3390/foods9111590
Kim JH, Kim YS, Kim TI, Li W, Mun J-G, Jeon HD, Kee J-Y, Choi J-G, Chung H-S. Unripe Black Raspberry (Rubus coreanus Miquel) Extract and Its Constitute, Ellagic Acid Induces T Cell Activation and Antitumor Immunity by Blocking PD-1/PD-L1 Interaction. Foods. 2020; 9(11):1590. https://doi.org/10.3390/foods9111590
Chicago/Turabian StyleKim, Ji Hye, Young Soo Kim, Tae In Kim, Wei Li, Jeong-Geon Mun, Hee Dong Jeon, Ji-Ye Kee, Jang-Gi Choi, and Hwan-Suck Chung. 2020. "Unripe Black Raspberry (Rubus coreanus Miquel) Extract and Its Constitute, Ellagic Acid Induces T Cell Activation and Antitumor Immunity by Blocking PD-1/PD-L1 Interaction" Foods 9, no. 11: 1590. https://doi.org/10.3390/foods9111590