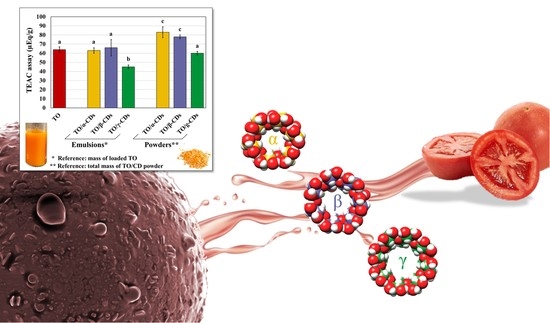
Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tomato Oil Encapsulation Process
2.3. Carotenoid Entrapment Efficiency (EE%)
2.4. Characterization of TO/α-, β-, and γ-CD Emulsions and Powders
2.4.1. Fourier Transform Infrared-Attenuated Total Reflection (FTIR-ATR) and Differential Scanning Calorimetry (DSC) Analyses
2.4.2. Scanning Electron Microscopy (SEM) and Laser Confocal Scanning Microscopy (LCSM)
2.5. Antioxidant Activity Measurements
2.6. Storage of TO/CD Emulsions and Powders
2.7. Kinetic Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Entrapment Efficiency of Carotenoids in the TO/CD Powders
3.2. Characterization of the TO/CD Emulsions and Powders
3.2.1. Macroscopic Characteristics of TO/α-, β-, and γ-CD Emulsions and Powders
3.2.2. LCSM and SEM Characterization of TO/CD Emulsions
3.2.3. FTIR-ATR
3.2.4. DSC
3.3. Antioxidant Activity of the TO/CD Emulsions and Powders
3.4. Effect of Storage Conditions on Carotenoid Shelf-Life Stability and Degradation Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martí, R.; Valcárcel, M.; Roselló, S.; Cebolla-Cornejo, J. Functional and health-promoting properties of tomatoes: It’s not just lycopene. In Tomato Chemistry, Industrial Processing and Product Development, 1st ed.; Porretta, S., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 285–303. [Google Scholar]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Rao, L.G.; MacKinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos. Int. 2006, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Ilharco, L.M.; Pagliaro, M. Lycopene: Emerging Production Methods and Applications of a Valued Carotenoid. ACS Sustain. Chem. Eng. 2016, 4, 643–650. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Laddomada, B.; Mita, G.; Caretto, S. Effects of Sodium Alginate Bead Encapsulation on the Storage Stability of Durum Wheat (Triticum durum Desf.) Bran Oil Extracted by Supercritical CO2. J. Agric. Food Chem. 2012, 60, 10689–10695. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Lenucci, M.S.; D’Amico, L.; Piro, G.; Mita, G. Effect of drying and co-matrix addition on the yield and quality of supercritical CO2 extracted pumpkin (Cucurbita moschata Duch.) oil. Food Chem. 2014, 148, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Siddiqui, M.W.; Tlili, I.; Montefusco, A.; Piro, G.; Hdider, C.; Lenucci, M.S. When Color Really Matters: Horticultural Performance and Functional Quality of High-Lycopene Tomatoes. Crit. Rev. Plant Sci. 2018, 37, 15–53. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Marrese, P.P.; Rizzi, V.; De Caroli, M.; Piro, G.; Fini, P.; Russo, G.L.; Mita, G. α-Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Bilotto, S.; Spagnuolo, C.; Durante, M.; Lenucci, M.S.; Mita, G.; Volpe, M.G.; Aquino, R.P.; Russo, G.L. A Carotenoid Extract from a Southern Italian Cultivar of Pumpkin Triggers Nonprotective Autophagy in Malignant Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Bruno, A.; Durante, M.; Marrese, P.P.; Migoni, D.; Laus, M.N.; Pace, E.; Pastore, D.; Mita, G.; Piro, G.; Lenucci, M.S. Shades of red: Comparative study on supercritical CO2 extraction of lycopene-rich oleoresins from gac, tomato and watermelon fruits and effect of the α-cyclodextrin clathrated extracts on cultured lung adenocarcinoma cells’ viability. J. Food Compos. Anal. 2018, 65, 23–32. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef] [PubMed]
- Moccia, S.; Russo, M.; Durante, M.; Lenucci, M.S.; Mita, G.; Russo, G.L. A carotenoid-enriched extract from pumpkin delays cell proliferation in a human chronic lymphocytic leukemia cell line through the modulation of autophagic flux. Curr. Res. Biotechnol. 2020, 2, 74–82. [Google Scholar] [CrossRef]
- Scita, G. [16] Stability of β-carotene under different laboratory conditions. Methods Enzymol. 1992, 213, 175–185. [Google Scholar] [CrossRef]
- Chasse, G.A.; Mak, M.L.; Deretey, E.; Farkas, I.; Torday, L.L.; Papp, J.G.; Sarma, D.S.R.; Agarwal, A.; Chakravarthi, S.; Agarwal, S.; et al. An ab initio computational study on selected lycopene isomers. J. Mol. Struct. Theochem. 2001, 571, 27–37. [Google Scholar] [CrossRef]
- Mele, A.; Mendichi, R.; Selva, A.; Molnar, P.; Tóth, G. Non-covalent associations of cyclomaltooligosaccharides (cyclodextrins) with carotenoids in water. A study on the α- and β-cyclodextrin/ψ,ψ-carotene (lycopene) systems by light scattering, ionspray ionization and tandem mass spectrometry. Carbohydr. Res. 2002, 337, 1129–1136. [Google Scholar] [CrossRef]
- Mastrogiacomo, D.; Lenucci, M.S.; Bonfrate, V.; Di Carolo, M.; Piro, G.; Valli, L.; Rescio, L.; Milano, F.; Comparelli, R.; De Leo, V.; et al. Lipid/detergent mixed micelles as a tool for transferring antioxidant power from hydrophobic natural extracts into bio-deliverable liposome carriers: The case of lycopene rich oleoresins. RSC Adv. 2015, 5, 3081–3093. [Google Scholar] [CrossRef]
- Ashraf, W.; Latif, A.; Zhang, L.; Jian, Z.; Chenqiang, W.; Rehman, A.; Hussain, A.; Siddiquy, M.; Karim, A. Technological Advancement in the Processing of Lycopene: A Review. Food Rev. Int. 2020, 1–27. [Google Scholar] [CrossRef]
- Saini, R.K.; Bekhit, A.E.-D.A.; Roohinejad, S.; Rengasamy, K.R.R.; Keum, Y.-S. Chemical Stability of Lycopene in Processed Products: A Review of the Effects of Processing Methods and Modern Preservation Strategies. J. Agric. Food Chem. 2019, 68, 712–726. [Google Scholar] [CrossRef]
- Vergallo, C. Nutraceutical Vegetable Oil Nanoformulations for Prevention and Management of Diseases. Nanomaterials 2020, 10, 1232. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. Nanocapsule formation by cyclodextrins. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries, 1st ed.; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 7; pp. 187–261. [Google Scholar]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–36. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Parmar, V.; Patel, G.; Abu-Thabit, N.Y. Responsive cyclodextrins as polymeric carriers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, 1st ed.; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2018; Volume 1, pp. 555–580. [Google Scholar]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-cyclodextrin cavity: Amount, stability and mechanism of binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P.J. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Blanch, G.P.; Del Castillo, M.L.R.; Caja, M.D.M.; Pérez-Méndez, M.; Sánchez-Cortés, S. Stabilization of all-trans-lycopene from tomato by encapsulation using cyclodextrins. Food Chem. 2007, 105, 1335–1341. [Google Scholar] [CrossRef] [Green Version]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A.; Loftsson, T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2012, 428, 152–163. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Wu, L.; Liao, Z.; Liu, M.; Yin, X.; Li, X.; Wang, M.; Lu, X.; Lv, N.; Singh, V.; He, Z.; et al. Fabrication of non-spherical Pickering emulsion droplets by cyclodextrins mediated molecular self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 163–172. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Hashizaki, K.; Taguchi, H.; Saito, Y. Formation and Characterization of Emulsions Using β-Cyclodextrin as an Emulsifier. Chem. Pharm. Bull. 2008, 56, 668–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathapa, B.G.; Paunov, V.N. Cyclodextrin stabilised emulsions and cyclodextrinosomes. Phys. Chem. Chem. Phys. 2013, 15, 17903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, H.; Tanaka, H.; Hashizaki, K.; Saito, Y.; Fujii, M. Application of Pickering Emulsion with Cyclodextrin as an Emulsifier to a Transdermal Drug Delivery Vehicle. Biol. Pharm. Bull. 2019, 42, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Lenucci, M.S.; Caccioppola, A.; Durante, M.; Serrone, L.; Leonardo, R.; Piro, G.; Dalessandro, G. Optimisation of biological and physical parameters for lycopene supercritical CO2 extraction from ordinary and high-pigment tomato cultivars. J. Sci. Food Agric. 2010, 90, 1709–1718. [Google Scholar] [CrossRef]
- Lenucci, M.S.; De Caroli, M.; Marrese, P.P.; Iurlaro, A.; Rescio, L.; Böhm, V.; Dalessandro, G.; Piro, G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015, 170, 193–202. [Google Scholar] [CrossRef]
- Piyawan, Y.; Varipat, A. Effects of type and ratio of carrier on physicochemical properties of microcapsules containing Gac fruit aril. Asia Pac. J. Sci. Technol. 2019, 24, 1–8. [Google Scholar]
- Gomes, L.; Petito, N.; Costa, V.; Falcao, D.; Araujo, K. Inclusion complexes of red bell pepper pigments with beta-cyclodextrin: Preparation, characterisation and application as natural colorant in yogurt. Food Chem. 2014, 148, 428–436. [Google Scholar] [CrossRef]
- De Lima Petito, N.; da Silva Dias, D.; Costa, V.G.; Falcão, D.Q.; de Lima Araujo, K.G. Increasing solubility of red bell pepper carotenoids by complexation with 2-hydroxypropyl-b-cyclodextrin. Food Chem. 2016, 208, 124–131. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on microencapsulation of lycopene by spray-drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, C.-X.; Lagoin, C.; Hai, M.; Arriaga, L.R.; Koehler, S.; Abbaspourrad, A.; Weitz, D.A. Dispersing hydrophobic natural colourant β-carotene in shellac particles for enhanced stability and tunable colour. R. Soc. Open Sci. 2017, 4, 170919. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hashizaki, K.; Taguchi, H.; Saito, Y. Preparation and characterization of n-alkane/water emulsion stabilized by cyclodextrin. J. Oleo Sci. 2009, 58, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Hashizaki, K.; Taguchi, H.; Saito, Y. Emulsifying Ability of β-Cyclodextrins for Common Oils. J. Dispers. Sci. Technol. 2010, 31, 1648–1651. [Google Scholar] [CrossRef]
- Hamoudi, M.C.; Bourasset, F.; Domergue-Dupont, V.; Gueutin, C.; Nicolas, V.; Fattal, E.; Bochot, A. Formulations based on alpha cyclodextrin and soybean oil: An approach to modulate the oral release of lipophilic drugs. J. Control. Release 2012, 161, 861–867. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Xiao, Q.; Wang, L.; Wang, M.; Lu, X.; York, P.; Shi, S.; Zhang, J. Two-way effects of surfactants on Pickering emulsions stabilized by the self-assembled microcrystals of α-cyclodextrin and oil. Phys. Chem. Chem. Phys. 2014, 16, 14059–14069. [Google Scholar] [CrossRef]
- Diaz-Salmeron, R.; Chaab, I.; Carn, F.; Djabourov, M.; Bouchemal, K. Pickering emulsions with α-cyclodextrin inclusions: Structure and thermal stability. J. Colloid Interface Sci. 2016, 482, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, K.; Kawano, K.; Ishii, J.; Nakamura, T. Structure of Inclusion Complexes of Cyclodextrins with Triglyceride at Vegetable Oil/Water Interface. J. Food Sci. 1992, 57, 655–656. [Google Scholar] [CrossRef]
- Leclercq, L.; Nardello-Rataj, V. Pickering emulsions based on cyclodextrins: A smart solution for antifungal azole derivatives topical delivery. Eur. J. Pharm. Sci. 2016, 82, 126–137. [Google Scholar] [CrossRef]
- Tervoort, E.; Studart, A.R.; Denier, C.; Gauckler, L.J. Pickering emulsions stabilized by in situ grown biologically active alkyl gallate microneedles. RSC Adv. 2012, 2, 8614–8618. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Specogna, E.; Li, K.W.; Djabourov, M.; Carn, F.; Bouchemal, K. Dehydration, Dissolution, and Melting of Cyclodextrin Crystals. J. Phys. Chem. B 2015, 119, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Giordano, F.; Novák, C.; Moyano, J.R. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Furlanetto, S.; Pinzauti, S. Differential scanning calorimetry as an analytical tool in the study of drug-cyclodextrin interactions. J. Therm. Anal. Calorim. 2003, 73, 635–646. [Google Scholar] [CrossRef]
- Galvão, J.G.; Silva, V.F.; Ferreira, S.G.; França, F.R.M.; Santos, D.A.; Freitas, L.S.; Alves, P.B.; Araújo, A.A.S.; Cavalcanti, S.C.H.; Nunes, R.S. β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochim. Acta 2015, 608, 14–19. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Li, X.; Zheng, Y. Inclusion complex of nateglinide with sulfobutyl ether β-cyclodextrin: Preparation, characterization and water solubility. J. Mol. Struct. 2017, 1141, 328–334. [Google Scholar] [CrossRef]
- Amruta, T.; Nancy, P.; Prashant, K.; Niteshkumar, S. Encapsulation of boswellic acid with β- and hydroxypropyl-β-cyclodextrin: Synthesis, characterization, in vitro drug release and molecular modelling studies. J. Mol. Struct. 2018, 1154, 504–510. [Google Scholar]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; Hădărugă, D.I. Thermal Analyses of Cyclodextrin Complexes. In Cyclodextrin Fundamentals, Reactivity and Analysis, 1st ed.; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 16, pp. 155–221. [Google Scholar]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Microencapsulated Antimicrobial Compounds as a Means to Enhance Electron Beam Irradiation Treatment for Inactivation of Pathogens on Fresh Spinach Leaves. J. Food Sci. 2011, 76, E479–E488. [Google Scholar] [CrossRef]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Pinto, L.M.A.; Fraceto, L.F.; Santana, M.H.A.; Pertinhez, T.A.; Junior, S.O.; De Paula, E. Physico-chemical characterization of benzocaine-β-cyclodextrin inclusion complexes. J. Pharm. Biomed. Anal. 2005, 39, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, M.A.; Alves, R.D.S.; Menezes, P.D.P.; Serafini, M.R.; Araújo, A.A.D.S.; Bezerra, D.P.; Quintans-Júnior, L.J. Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β-cyclodextrin, improves the pharmacological response on cancer pain experimental protocols. Chem. Biol. Interact. 2015, 227, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. “Surface water” and “strong-bonded water” in cyclodextrins: A Karl Fischer titration approach. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 297–302. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Kabirov, D.; Beregova, D.; Gamov, G.; Sharnin, V.; Biondi, M.; Mayol, L.; D’Aria, F.; Giancola, C. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J. Therm. Anal. Calorim. 2019, 138, 417–424. [Google Scholar] [CrossRef]
- Serri, C.; Argirò, M.; Piras, L.; Mita, D.G.; Saija, A.; Mita, L.; Forte, M.; Giarra, S.; Biondi, M.; Crispi, S.; et al. Nano-precipitated curcumin loaded particles: Effect of carrier size and drug complexation with (2-hydroxypropyl)-β-cyclodextrin on their biological performances. Int. J. Pharm. 2017, 520, 21–28. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P. The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. LWT Food Sci. Technol. 2017, 85, 517–523. [Google Scholar] [CrossRef]
- Przybysz, M.A.; Szterk, A.; Symoniuk, E.; Gąszczyk, M.; Dłużewska, E. α- and β-Carotene Stability During Storage of Microspheres Obtained from Spray-Dried Microencapsulation Technology. Pol. J. Food Nutr. Sci. 2018, 68, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of Spray-drying, Drum-drying and Freeze-drying for β-Carotene Encapsulation and Preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S.; Roos, Y.H. Microstructure formation of maltodextrin and sugar matrices in freeze-dried systems. Carbohydr. Polym. 2012, 88, 734–742. [Google Scholar] [CrossRef]
- Haas, K.; Robben, P.; Kiesslich, A.; Volkert, M.; Jäger, H. Stabilization of Crystalline Carotenoids in Carrot Concentrate Powders: Effects of Drying Technology, Carrier Material, and Antioxidants. Foods 2019, 8, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri-Rigi, A.; Abbasi, S. Lycopene microemulsion storability: Monitoring colour and rheological properties. Int. Nano Lett. 2020, 10, 119–129. [Google Scholar] [CrossRef]
- Wagner, L.A.; Warthesen, J.J. Stability of Spray-Dried Encapsulated Carrot Carotenes. J. Food Sci. 1995, 60, 1048–1053. [Google Scholar] [CrossRef]
- Dłużewska, E.; Florowska, A.; Domian, E.; Wojciechowska, M.; Maszewska, M. The Influence of the Agglomeration Process on Stability of Microencapsulated β-Carotene. Int. J. Food Eng. 2019, 16. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Liu, Y.L.; Zhu, X.W.; Pan, S.Y.; Wang, L.F. Encapsulation of tomato oleoresin with zein prepared from corn gluten meal. J. Food Eng. 2013, 119, 439–445. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Chiu, C.P.; Chien, J.T.; Ho, G.H.; Yang, J.; Chen, B.H. Encapsulation of lycopene extract from tomato pulp waste with gelatin and poly(gamma-glutamic acid) as carrier. J. Agric. Food Chem. 2007, 55, 5123–5130. [Google Scholar] [CrossRef]
- Robert, P.; Carlsson, R.M.; Romero, N.; Masson, L. Stability of spray-dried encapsulated carotenoid pigments from rosa mosqueta (Rosa rubiginosa) oleoresin. J. Am. Oil Chem. Soc. 2003, 80, 1115–1120. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. A storage study of encapsulated gac (Momordica cochinchinensis) oil powder and its fortification into foods. Food Bioprod. Process. 2015, 96, 113–125. [Google Scholar] [CrossRef]










| Carotenoids | TO | Powders | ||
|---|---|---|---|---|
| TO/α-CDs | TO/β-CDs | TO/γ-CDs | ||
| mg/100 g | EE% (mg/100 g Oil) | EE% (mg/100 g Oil) | EE% (mg/100 g Oil) | |
| β-carotene | 34.1 ± 2.3 a | 96.8 (33.0 ± 1.2 a) | 97.4 (33.2 ± 1.2 a) | 68 (23.2 ± 1.7 b) |
| [Z]-lycopene isomers | 229.2 ± 2.8 a | 76.5 (175.3 ± 3.2 b) | 71.5 (163.9 ± 2.9 c) | 42 (96.1 ± 2.3 d) |
| All-[E]-lycopene | 615.4 ± 31.1 a | 54.0 (332.3 ± 16.2 b) | 57.0 (350.8 ± 12.2 b) | 44 (270.9 ± 6.5 c) |
| Total | 878.7 ± 35.5 a | 61.5 (540.2 ± 10.6 b) | 62.4 (547.9 ± 16.3 b) | 44 (390.2 ± 10.5 c) |
| Samples | TEAC µEq/g TO (µEq/g Powder) |
|---|---|
| TO | 64 ± 3 bc |
| Emulsions | |
| TO/α-CDs | 75 ± 5 ab |
| TO/β-CDs | 73 ± 5 ab |
| TO/γ-CDs | 54 ± 4 c |
| Powders | |
| TO/α-CDs | 85 ± 9 a (29 ± 3 A) |
| TO/β-CDs | 72 ± 6 ab (22 ± 2 B) |
| TO/γ-CDs | 68 ± 6 bc (19 ± 2 B) |
| Samples | Temp (°C) | β-Carotene | [Z]-Lycopene Isomers | All-[E]-Lycopene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r2 | k (10−3 d−1) | t1/2 (d) | r2 | k (10−3 d−1) | t1/2 (d) | r2 | k (10−3 d−1) | t1/2 (d) | ||
| TO | 25 | 0.74 | 13.5 | 51 | 0.76 | 23.0 | 30 | 0.91 | 5.1 | 136 |
| 4 | 0.85 | 11.9 | 58 | 0.92 | 16.0 | 43 | 0.94 | 4.4 | 158 | |
| Emulsions | ||||||||||
| TO/α-CDs | 25 | 0.93 | 59.9 | 12 | 0.92 | 60.4 | 11 | 0.93 | 37.7 | 18 |
| 4 | 0.85 | 1.9 | 365 | 0.95 | 13.2 | 52 | 0.96 | 28.3 | 24 | |
| TO/β-CDs | 25 | 0.94 | 56.8 | 12 | 0.90 | 61.5 | 11 | 0.98 | 18.0 | 38 |
| 4 | 0.71 | 1.1 | 608 | 0.89 | 5.9 | 117 | 0.93 | 16.5 | 42 | |
| TO/γ-CDs | 25 | 0.91 | 18.2 | 38 | 0.94 | 18.5 | 37 | 0.93 | 21.0 | 33 |
| 4 | 0.77 | 6.1 | 113 | 0.76 | 33.9 | 21 | 0.96 | 23.3 | 30 | |
| Powders | ||||||||||
| TO/α-CD | 25 | 0.93 | 60.6 | 11 | 0.93 | 23.1 | 30 | 0.90 | 58 | 12 |
| 4 | 0.77 | 3.8 | 180 | 0.97 | 13.3 | 52 | 0.99 | 24.8 | 28 | |
| TO/β-CD | 25 | 0.81 | 56.9 | 12 | 0.76 | 62 | 11 | 0.80 | 64 | 11 |
| 4 | 0.75 | 5.1 | 137 | 0.95 | 20.2 | 34 | 0.99 | 28.1 | 25 | |
| TO/γ-CD | 25 | 0.77 | 28.2 | 25 | 0.99 | 18.5 | 36 | 0.99 | 21.0 | 33 |
| 4 | 0.94 | 3.4 | 205 | 0.93 | 4.7 | 146 | 0.95 | 20.6 | 34 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durante, M.; Milano, F.; Caroli, M.D.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability. Foods 2020, 9, 1553. https://doi.org/10.3390/foods9111553
Durante M, Milano F, Caroli MD, Giotta L, Piro G, Mita G, Frigione M, Lenucci MS. Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability. Foods. 2020; 9(11):1553. https://doi.org/10.3390/foods9111553
Chicago/Turabian StyleDurante, Miriana, Francesco Milano, Monica De Caroli, Livia Giotta, Gabriella Piro, Giovanni Mita, Mariaenrica Frigione, and Marcello Salvatore Lenucci. 2020. "Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability" Foods 9, no. 11: 1553. https://doi.org/10.3390/foods9111553