Effects of Probiotic Supplementation on Dyslipidemia in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Data Items and Data Collection Process
2.3. Assessment of Risk of Bias
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics of Included Papers
3.2. Risk of Bias in Individual Studies
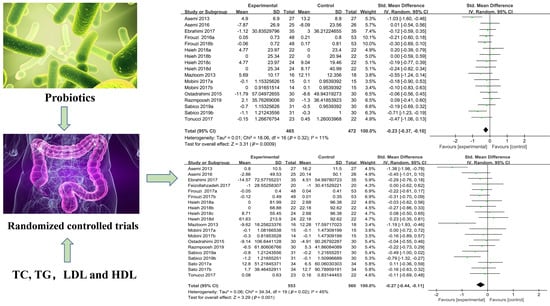
3.3. Effects of Probiotics on Blood Lipid Profiles
3.3.1. Plasma TC Levels
3.3.2. Plasma TG Levels
3.3.3. Plasma LDL-C Levels
3.3.4. Plasma HDL-C Levels
3.4. Subgroup Analysis
3.5. Publication Bias
3.6. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tai, N.; Wong, F.S.; Wen, L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohail, M.U.; Althani, A.; Anwar, H.; Rizzi, R.; Marei, H.E. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J. Diabetes. Res. 2017, 2017, 9631435. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res. Care 2015, 3, e000093. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, G.; Sreehari, G.K.; Vijayakumar, A.; Jaleel, A. Distinct predictors and comorbidities in early onset type 2 diabetes mellitus among Asian Indians. Metab. Syndr. Relat. Disord. 2017, 15, 458–464. [Google Scholar] [CrossRef]
- Jisieike-Onuigbo, N.N.; Unuigbe, E.I.; Oguejiofor, C.O. Dyslipidemias in type 2 diabetes mellitus patients in Nnewi South-East Nigeria. Ann. Afr. Med. 2011, 10, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.C.; Millns, H.; Neil, H.A.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Oguejiofor, O.C.; Onwukwe, C.H.; Odenigbo, C.U. Dyslipidemia in Nigeria: Prevalence and pattern. Ann. Afr. Med. 2012, 11, 197–202. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Borén, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef]
- Taskinen, M.R. Type 2 diabetes as a lipid disorder. Curr. Mol. Med. 2005, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [Green Version]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Taskinen, M.R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboobi, S.; Iraj, B.; Maghsoudi, Z.; Feizi, A.; Ghiasvand, R.; Askari, G.; Maayeshi, N. The effects of probiotic supplementation on markers of blood lipids, and blood pressure in patients with prediabetes: A randomized clinical trial. Int. J. Prev. Med. 2014, 5, 1239–1246. [Google Scholar]
- Mann, G.V. A factor in yogurt which lowers cholesteremia in man. Atherosclerosis 1977, 26, 335–340. [Google Scholar] [CrossRef]
- Li, C.F.; Li, X.; Han, H.Q.; Cui, H.L.; Peng, M.; Wang, G.L.; Wang, Z.Q. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: A meta-analysis of randomized, controlled trials. Medicine 2016, 95, e4088. [Google Scholar] [CrossRef]
- Hendijani, F.; Akbari, V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin. Nutr. 2018, 37, 532–541. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ansari, M.G.A.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab. Syndr. 2019, 13, 175–182. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Deeks, J.J. Selecting studies and collecting data. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Green, S., Eds.; John Wiley & Sons: West Sussex, UK, 2008; pp. 151–190. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2011, 5, S38. [Google Scholar]
- Morgan, M. Meta-study of qualitative health research: A practical guide to meta-analysis and meta-synthesis. Nurs. Crit. Care 2003, 8, 184. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Interpretation of tests of heterogeneity and bias in meta-analysis. J. Eval. Clin. Pract. 2008, 14, 951–957. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ 2007, 176, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.A.; Shakeri, H.; Esmaillzadeh, A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef]
- Ebrahimi, Z.S.; Nasli-Esfahani, E.; Nadjarzade, A.; Mozaffari-Khosravi, H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: A randomized, double-blind, clinical trial. J. Diabetes Metab. Disord. 2017, 16, 23. [Google Scholar] [CrossRef] [Green Version]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V.; Akbarian-Moghari, A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Feizollahzadeh, S.; Ghiasvand, R.; Rezaei, A.; Khanahmad, H.; Sadeghi, A.; Hariri, M. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob. Proteins 2017, 9, 41–47. [Google Scholar] [CrossRef]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Gheshlaghi, Z.B.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Tsai, W.H.; Jheng, Y.P.; Su, S.L.; Wang, S.Y.; Lin, C.C.; Chen, Y.H.; Chang, W.W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Forslund, H.B.; Perkins, R.; Bäckhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 12115. [Google Scholar] [CrossRef] [Green Version]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.H.; Byrne, C.D.; Tzoulaki, I.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. Metabolic syndrome, haemostatic and inflammatory markers, cerebrovascular and peripheral arterial disease: The Edinburgh Artery Study. Atherosclerosis 2009, 203, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Colon, S.M.; Mo, J.; Duan, Y.; Liu, J.; Caulfield, J.E.; Jin, X.; Liao, D. Metabolic syndrome clusters and the risk of incident stroke: The atherosclerosis risk in communities (ARIC) study. Stroke 2009, 40, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Lüscher, T.F.; Landmesser, U.; von Eckardstein, A.; Fogelman, A.M. High-density lipoprotein: Vascular protective effects, dysfunction, and potential as therapeutic target. Circ. Res. 2014, 114, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T. Pathophysiology of diabetic dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soran, H.; Dent, R.; Durrington, P. Evidence-based goals in LDL-C reduction. Clin. Res. Cardiol. 2017, 106, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Liu, X.M.; Zhang, Q.X.; Tian, F.W.; Zhang, H.; Zhang, H.P.; Chen, W. Research advances with regards to clinical outcome and potential mechanisms of the cholesterol-lowering effects of probiotic. Clin. Lipidol. 2012, 7, 501–507. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Farnworth, E.R.; Jones, P.J. Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am. J. Clin. Nutr. 2000, 71, 674–681. [Google Scholar] [CrossRef]
- Hu, Y.M.; Zhou, F.; Yuan, Y.; Xu, Y.C. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med. Clin. 2017, 148, 362–370. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Lewis, J.R.; Thompson, P.L.; Prince, R.L. The effects of probiotic bacteria on glycaemic control in overweight men and women: A randomised controlled trial. Eur. J. Clin. Nutr. 2014, 68, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.C.U.; Manzoni, M.S.J.; Bedani, R.; Roselino, M.N.; Celiberto, L.S.; Vendramini, R.C.; de Valdez, G.F.; Abdalla, D.S.P.; Pinto, R.A.; Rosetto, D.; et al. Probiotic soy product supplemented with isoflavones improves the lipid profile of moderately hypercholesterolemic men: A randomized controlled trial. Nutrients 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Gilliland, S.E.; Nelson, C.R.; Maxwell, C. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1985, 49, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Liu, X.M.; Zhang, Q.X.; Shen, Z.; Tian, F.W.; Zhang, H.; Sun, Z.H.; Zhang, H.P.; Chen, W. Influence of consumption of probiotics on the plasma lipid profile: A meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Buttarazzi, I.; Kolida, S.; Quercia, S.; Baldini, J.; Swann, J.R.; Brigidi, P.; Gibson, G.R. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS ONE 2017, 12, e0187964. [Google Scholar] [CrossRef]
- Mahboobi, S.; Rahimi, F.; Jafarnejad, S. Effects of prebiotic and synbiotic supplementation on glycaemia and lipid profile in type 2 diabetes: A meta-analysis of randomized controlled trials. Adv. Pharm. Bull. 2018, 8, 565–574. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Baradar Jalili, R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. Daru 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: A meta-analysis of 12 randomized controlled trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, F.; Han, Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: A meta-analysis of RCTs. Medicine 2017, 96, e9166. [Google Scholar] [CrossRef]







| Author/Date | Age | No. of Participants (Intervention/Placebo) | Administered Probiotics | Whether the Included RCTs Have Measured Dyslipidemia Indicators | Type of Study | Trial Number | Country |
|---|---|---|---|---|---|---|---|
| Asemi et al., 2013 [29] | 35–70 | 60 (27/27) | Capsules: L. casei, 7 × 109 CFU; L. acidophilus, 2 × 109 CFU; L. rhamnosus, 1.5 × 109 CFU; B. breve, 2 × 1010 CFU; B. longum, 7 × 109 CFU; L. bulgaricus, 2 × 108 CFU; S. thermophilus, 1.5 × 109 CFU (8 weeks). | Yes | RCT | - | Iran |
| Asemi et al., 2014 [30] | 35–70 | 62 (31/31) | Synbiotic food: L. sporogenes, 1 × 107 CFU (each day for 6 weeks). | Yes | RCT | IRCT201201195623N1 | Iran |
| Asemi et al., 2016 [31] | 35–70 | 51 (25/26) | Synbiotic food: 1 × 107 CFU, L. sporogenes (three times a day for 6 weeks). | Yes | RCT | Iran | |
| Ebrahimi et al., 2017 [32] | 35–75 | 70 (35/35) | Capsules: Lactobacillus; Bifidobacterium family, S. thermophilus (500 mg/d for 9 weeks). | Yes | RCT | IRCT2015072223284N1 | Iran |
| Ejtahed et al., 2011 [33] | 30–60 | 60 (30/30) | Yogurt: L. acidophilus La5; B. lactis Bb12 (300 g/day for 6 weeks). | Yes | RCT | IRCT 138903223 533N1 | Iran |
| Feizollahzadeh et al., 2016 [34] | 35–68 | 40 (20/20) | Soy milk: L. plantarum A7, 2 × 107 CFU (200 mL milk/day for 8 weeks). | Yes (lack of TC level) | RCT | IRCT201405265062N8 | Iran |
| Firouzi et al., 2017 [37] | 30–70 | 101 (48/53) | Sachet: L. acidophilus, 3 × 1010; L. lactis, 3 × 1010; B. bifidum,3 × 1010; B. longum, 3 × 1010; L. casei, 3 × 1010; B. infantis, 3 × 1010 (1010 cfus/day for 12 weeks). | Yes | RCT | NCT01752 803 | Malaysia |
| Hsieh et al., 2018 [38] | 25–70 | 68 (46/22) | Capsules: L. reuteri ADR-1 or L. reuteir ADR-3, 2 × 109 CFU or 1 × 1010 cells (9 months). | Yes | RCT | NCT02274272 | China |
| Mazloom et al., 2013 [35] | 25–65 | 34 (16/18) | Capsules: L. bulgaricus, L. bifidum, L. acidophilus, L. casei (3000 mg/day for 6 weeks). | Yes | RCT | - | Iran |
| Mobini et al., 2017 [39] | 50–75 | 44 (15/high dose 14/low dose 15) | Probiotic powder: L. reuteri DSM 17938, low (108 CFU/day) or high dose (1010 CFU/day) (one dose per day for 12 weeks). | Yes | RCT | NCT01836796 | Sweden |
| Ostadrahimi et al. 2015 [36] | 35–65 | 60 (30/30) | Fermented milk: L. casei, L. acidophilus and Bifidobacteria (600 mL/day for 8 weeks). | Yes | RCT | IRCT2013 07092017N 14 | Iran |
| Razmpoosh et al., 2019 [20] | 30–75 | 60 (30/30) | Capsule: L. acidophilus, 2 × 109 CFU; L. rhamnosus, 1.5 × 109 CFU; L. casei 7 × 109 CFU; L. bulgaricus, 2 × 108 CFU; B. breve, 3 × 1010 CFU; B. longum, 7 × 109 CFU; S. thermophilus, 1.5 × 109 CFU (6 weeks). | Yes | RCT | IRCT2013100714925N1 | Iran |
| Sabico et al., 2019 [19] | 30–60 | 61 (31/30) | Freeze-dried powder: B. lactis W52, 2.5 × 109 cfu/g; L. acidophilus W37, 2.5 × 109 cfu/g; L. brevis W63, 2.5 × 109 cfu/g; B. bifidum W23, 2.5 × 109 cfu/g; L. casei W56, 2.5 × 109 cfu/g; L. salivarius W24, 2.5 × 109 cfu/g; L. lactis W58, 2.5 × 109 cfu/g; L. lactis W19 2.5 × 109 cfu/g (twice daily for 6 months). | Yes | RCT | NCT01765517 | Saudi Arabia |
| Sato et al., 2017 [40] | 30–79 | 68 (34/34) | Milk: L. casei, 4 × 1010(80-mL bottle milk fermented with one bottle of milk every day for 16 weeks). | Yes (lack of TC and LDL-C levels) | RCT | UMIN000018246 | Japan |
| Tonucci et al., 2015 [41] | 35–60 | 45 (23/22) | Fermented milk: B. lactis BB12 and L. acidophilus LA5 (6 weeks). | Yes | RCT | Ensaiosclini cos.gov.br/ rg/RBR-219644 | Brazil |
| Subgroup | TC | TG | LDL | HDL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Pooled Effect (95% CI) mmol/L | I2 | p | No | Pooled Effect (95% CI) mmol/L | I2 | p | No | Pooled Effect (95% CI) mmol/L | I2 | p | No | Pooled Effect (95% CI) mmol/L | I2 | p | |
| Overall analysis | 17 | −0.23 (−0.37, −0.10) | 11 | 0.0009 | 20 | −0.27 (−0.44, −0.11) | 45 | 0.001 | 18 | −0.11 (−0.26, 0.04) | 26 | 0.14 | 20 | 0.09 (−0.06, 0.24) | 36 | 0.22 |
| Number of strains | ||||||||||||||||
| <2 | 7 | −0.07 (−0.30, 0.16) | 0 | 0.58 | 10 | −0.06 (−0.24, 0.12) | 0 | 0.50 | 8 | −0.04 (−0.28, 0.20) | 16 | 0.75 | 10 | 0.03 (−0.15, 0.21) | 0 | 0.73 |
| ≥2 | 10 | −0.32 (−0.52, −0.12) | 34 | 0.001 | 10 | −0.46 (−0.71, −0.21) | 58 | 0.0003 | 10 | −0.16 (−0.36, 0.04) | 36 | 0.11 | 10 | 0.14 (−0.11, 0.39) | 60 | 0.28 |
| Duration (week) | ||||||||||||||||
| <8 | 5 | −0.18 (−0.41, 0.05) | 0 | 0.13 | 5 | −0.37 (−0.68, −0.07) | 38 | 0.02 | 5 | −0.06 (−0.29, 0.17) | 0 | 0.61 | 5 | 0.04 (−0.20, 0.27) | 0 | 0.77 |
| ≥8 | 12 | −0.25 (−0.43, −0.07) | 23 | 0.006 | 15 | −0.24 (−0.43, −0.04) | 49 | 0.02 | 13 | −0.13 (−0.32, 0.07) | 37 | 0.20 | 15 | 0.12 (−0.07, 0.31) | 46 | 0.23 |
| BMI | ||||||||||||||||
| <29 | 9 | −0.12 (−0.31, 0.06) | 0 | 0.19 | 12 | −0.13 (−0.29, 0.04) | 9 | 0.14 | 10 | −0.12 (−0.31, 0.07) | 10 | 0.21 | 12 | −0.01 (−0.16, 0.15) | 0 | 0.94 |
| ≥29 | 8 | −0.34 (−0.57, −0.11) | 33 | 0.003 | 8 | −0.48 (−0.75, −0.20) | 53 | 0.0006 | 8 | −0.10 (−0.36, 0.15) | 46 | 0.41 | 8 | 0.24 (−0.06, 0.54) | 61 | 0.12 |
| Type of intervention | ||||||||||||||||
| Liquid | 2 | −0.23 (−0.63, 0.16) | 5 | 0.25 | 5 | −0.04 (−0.27, 0.20) | 0 | 0.76 | 3 | −0.37 (−0.97, 0.23) | 69 | 0.22 | 5 | −0.00 (−0.27, 0.26) | 21 | 0.99 |
| Powder | 15 | −0.23 (−0.39, −0.08) | 18 | 0.003 | 15 | −0.36 (−0.56, −0.15) | 51 | 0.0005 | 15 | −0.07 (−0.21, 0.07) | 5 | 0.34 | 15 | 0.12 (−0.06, 0.30) | 40 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Probiotic Supplementation on Dyslipidemia in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Foods 2020, 9, 1540. https://doi.org/10.3390/foods9111540
Wang C, Zhang C, Li S, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. Effects of Probiotic Supplementation on Dyslipidemia in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Foods. 2020; 9(11):1540. https://doi.org/10.3390/foods9111540
Chicago/Turabian StyleWang, Chen, Chengcheng Zhang, Sijia Li, Leilei Yu, Fengwei Tian, Jianxin Zhao, Hao Zhang, Wei Chen, and Qixiao Zhai. 2020. "Effects of Probiotic Supplementation on Dyslipidemia in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials" Foods 9, no. 11: 1540. https://doi.org/10.3390/foods9111540