Real-Time Monitoring of Volatile Compounds Losses in the Oven during Baking and Toasting of Gluten-Free Bread Doughs: A PTR-MS Evidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Standards
2.2. Preparation of Standard Solutions
2.3. Gluten-Free and Wheat Bread Ingredients
2.4. Gluten-Free Bread Making
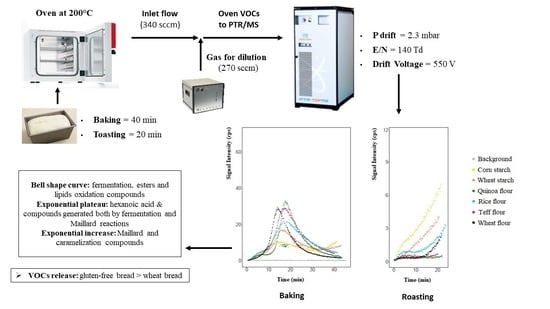
2.5. On-Line Monitoring of Volatile Compounds during Baking and Toasting of Gluten-Free and Wheat Control Breads by PTR-ToF-MS
2.6. Bread Moisture Loss
2.7. Statistical Analysis
3. Results and Discussion
3.1. On-Line Monitoring of Volatile Compounds during Bread Baking by PTR-ToF-MS: General Patterns
3.2. On-Line Monitoring of Volatile Compounds during Bread Toasting by PTR-ToF-MS: General Patterns
3.3. Differences in the Extent of Release of Volatile Compounds during Baking and Toasting Based on the Type of Flour: Gluten-Free and Wheat Control Breads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| E/N | electric field to gas density ratio |
| fast-GC | fast gas chromatography |
| FC | flow controller |
| HPMC | hydroxy propyl methyl cellulose |
| m/z | mass to charge ratio |
| PTFE | polytetrafluoroethylene |
| PTR-ToF-MS | proton transfer reaction-time of flight-mass spectrometry |
| sccm | standard cubic centimetres per minute |
References
- Makhoul, S.; Romano, A.; Cappellin, L.; Spano, G.; Capozzi, V.; Benozzi, E.; Märk, T.D.; Aprea, E.; Gasperi, F.; El-Nakat, H.; et al. Proton-transfer-reaction mass spectrometry for the study of the production of volatile compounds by bakery yeast starters. J. Mass Spectrom. 2014, 49, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Guinet, R.; Godon, B. La Panification Française, 1st ed.; Lavoisier: Paris, France, 1996. [Google Scholar]
- Paraskevopoulou, A.; Chrysanthou, A.; Koutidou, M. Characterisation of volatile compounds of lupin protein isolate-enriched wheat flour bread. Food Res. Int. 2012, 48, 568–577. [Google Scholar] [CrossRef]
- Pico, J.; Antolín, B.; Román, L.; Gómez, M.; Bernal, J. Analysis of volatile compounds in gluten-free bread crusts with an optimised and validated SPME-GC/QTOF methodology. Food Res. Int. 2018, 106, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Inoue, M.; Araki, T.; Iwabuchi, H.; Sagara, Y. Odorant transfer characteristics of white bread during baking. Biosci. Biotechnol. Biochem. 2011, 75, 261–267. [Google Scholar] [CrossRef]
- Pacyński, M.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Improving the aroma of gluten-free bread. LWT Food Sci. Technol. 2015, 63, 706–713. [Google Scholar] [CrossRef]
- Poinot, P.; Arvisenet, G.; Grua-Priol, J.; Fillonneau, C.; Le Bail, A.; Prost, C. Influence of inulin on bread: Kinetics and physico-chemical indicators of the formation of volatile compounds during baking. Food Chem. 2010, 119, 1474–1484. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef]
- Heenan, S.P.; Dufour, J.P.; Hamid, N.; Harvey, W.; Delahunty, C.M. Characterisation of fresh bread flavour: Relationships between sensory characteristics and volatile composition. Food Chem. 2009, 116, 249–257. [Google Scholar] [CrossRef]
- Capozzi, V.; Makhoul, S.; Aprea, E.; Romano, A.; Cappellin, L.; Jimena, A.S.; Spano, G.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-MS characterisation of VOCs associated with commercial aromatic bakery yeasts of wine and beer origin. Molecules 2016, 21, 483. [Google Scholar] [CrossRef]
- Makhoul, S.; Romano, A.; Capozzi, V.; Spano, G.; Aprea, E.; Cappellin, L.; Benozzi, E.; Scampicchio, M.; Märk, T.D.; Gasperi, F.; et al. Volatile compound production during the bread-making process: Effect of flour, yeast and their interaction. Food Bioprocess Technol. 2015, 8, 1925–1937. [Google Scholar] [CrossRef]
- Jourdren, S.; Masson, M.; Saint-Eve, A.; Panouillé, M.; Blumenthal, D.; Lejeune, P.; Déléris, I.; Souchon, I. Effect of bread crumb and crust structure on the in vivo release of volatiles and the dynamics of aroma perception. J. Agric. Food Chem. 2017, 65, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Jourdren, S.; Saint-Eve, A.; Pollet, B.; Panouillé, M.; Lejeune, P.; Guichard, E.; Déléris, I.; Souchon, I. Gaining deeper insight into aroma perception: An integrative study of the oral processing of breads with different structures. Food Res. Int. 2017, 92, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Inoue, M.; Araki, T.; Iwabuchi, H.; Sagara, Y. A PTR-MS-based protocol for simulating bread aroma during mastication. Food Bioprocess Technol. 2012, 5, 1228–1237. [Google Scholar] [CrossRef]
- Pico, J.; Khomenko, I.; Capozzi, V.; Navarini, L.; Bernal, J.; Gómez, M.; Biasioli, F. Analysis of volatile organic compounds in crumb and crust of different baked and toasted gluten-free breads by direct PTR—ToF—MS and fast—GC—PTR—ToF—MS. J. Mass Spectrom. 2018, 53, 893–902. [Google Scholar] [CrossRef]
- Capozzi, V.; Yener, S.; Khomenko, I.; Farneti, B.; Cappelin, L.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-ToF-MS Coupled with an automated sampling system and tailored data analysis for food studies: Bioprocess monitoring, screening and nose-space analysis. JOVE 2017, 123, 54075. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. Aroma of wheat bread crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- Pico, J.; Martínez, M.M.; Bernal, J.; Gómez, M. Evolution of volatile compounds in gluten-free bread: From dough to crumb. Food Chem. 2017, 227, 179–186. [Google Scholar] [CrossRef]
- Gardner, H.W. Decomposition of linoleic acid hydroperoxides. Enzymic reactions compared with nonenzymic. J. Agric. Food Chem. 1975, 23, 129–136. [Google Scholar] [CrossRef]
- Ramey, D.D.; Ough, C.S. Volatile ester hydrolysis or formation during storage of model solutions and wines. J. Agric. Food Chem. 1980, 28, 928–934. [Google Scholar] [CrossRef]
- Arvisenet, G.; Le Bail, P.; Voilley, A.; Cayot, N. Influence of physicochemical interactions between amylose and aroma compounds on the retention of aroma in food-like matrices. J. Agric. Food Chem. 2002, 50, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2007, 18, 49–70. [Google Scholar] [CrossRef]
- Onishi, M.; Inoue, M.; Araki, T.; Iwabuchi, H.; Sagara, Y. Characteristic coloring curve for white bread during baking. Biosci. Biotechnol. Biochem. 2011, 75, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graus, M.; Müller, M.; Hansel, A. High Resolution PTR-TOF: Quantification and formula Confirmation of VOC in real time. J. Am. Soc. Mass Spectrom. 2010, 21, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jeckle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 4), 240–257. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Hirose, Y.; Fujita, T.; Ishii, T.; Ueno, N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010, 119, 1300–1306. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.L.; Gómez, M. Influence of different flours and starches on gluten-free bread aroma. J. Food Sci. Technol. 2017, 54, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Pico, J.; Antolín, B.; Román, L.; Bernal, J.; Gómez, M. Selection of the most suitable mixture of flours and starches for the improvement of gluten-free breads through their volatile profiles. Eur. Food Res. Technol. 2019, 245, 1755–1766. [Google Scholar] [CrossRef]
- Pico, J.; Hansen, Å.S.; Petersen, M.A. Comparison of the volatile profiles of the crumb of gluten-free breads by DHE-GC/MS. J. Cereal Sci. 2017, 76, 280–288. [Google Scholar] [CrossRef]





| Volatile Compound | m/z | Formula | Identification |
|---|---|---|---|
| 3-Methyl-1-butanol | 71.0856 | C5H11+ | Standard (1) |
| 2-Methyl-1-butanol | Standard (2) | ||
| 1-Pentanol | Standard (3) | ||
| Hexanal | 83.0863 | C6H11+ | Standard (4) |
| 1-Hexanol | 85.1010 | C6H13+ | Standard (5) |
| Furfural | 97.0288 | C5H5O2+ | Standard (6) |
| 5-Methylfurfural | 111.0451 | C6H7O2+ | Standard (7) |
| Pyrazine | 81.0369 | C4H5N2+ | Standard (8) |
| 2,3-Butanedione | 87.0443 | C4H7O2+ | Standard (9) |
| Phenylacetaldehyde | 121.0680 | C8H9O+ | Standard (10) |
| 2-Ethyl-3-methylpyrazine | 123.0826 | C7H11N2+ | Standard (11) |
| 2-Acetylpyrazine | Standard (12) | ||
| 2-Ethylpyrazine | 109.0711 | C6H9N2+ | Standard (13) |
| 2,3-Dimethylpyrazine | Standard (14) | ||
| 2,5-Dimethylpyrazine | Standard (15) | ||
| 2,6-Dimethylpyrazine | Standard (16) | ||
| Benzyl alcohol | C7H9O+ | Standard (17) | |
| Butanoic acid | 89.0599 | C4H9O2+ | Standard (18) |
| Heptanal | 97.1028 | C7H13+ | Standard (19) |
| 1-Octen-3-ol | 111.1181 | C8H15+ | Standard (20) |
| 2-Acetyl-1-pyrroline | 112.0759 | C6H10NO+ | Standard (21) |
| 2,3-Diethylpyrazine | 137.0983 | C8H13N2+ | Standard (22) |
| Nonanal | 125.1339 | C9H17+ | Standard (23) |
| 2,4-(E,E)-Decadienal | 135.1221 | C10H15+ | Standard (24) |
| Ethyl octanoate | 173.1577 | C10H21O2+ | Standard (25) |
| Ethyl hexanoate | 145.1239 | C8H17O2+ | Standard (26) |
| Ethyl hexanoate | 145.1239 | C8H17O2+ | Standard (27) |
| 1-Methylpyrrol | 82.0700 | C5H8N+ | Standard (28) |
| Limonene | 137.1354 | C10H17+ | Standard (29) |
| 3-Penten-2-ol | 69.0702 | C5H9+ | Standard (30) |
| Benzaldehyde | 107.0518 | C7H7O+ | Standard (31) |
| Hexyl acetate | 145.1239 | C8H17O2+ | Standard (32) |
| Acetic acid | 61.0280 | C2H5O2+ | Standard (33) |
| Furfuryl alcohol | 81.0369 | C5H5O+ | Standard (34) |
| Acetoin | 89.0599 | C4H9O2+ | Standard (35) |
| 3-Methylbutanoic acid | 103.0704 | C5H11O2+ | Standard (36) |
| 2-Methylbutanoic acid | 103.0704 | C5H11O2+ | Standard (37) |
| Phenylethyl alcohol | 105.0721 | C8H9+ | Standard (38) |
| Hexanoic acid | 117.0921 | C6H13O2+ | Standard (39) |
| Furan | 69.0334 | C4H4OH+ | Tentative |
| 3-Methylbutanal | 69.0702 | C5H9+ | Tentative |
| 2-Methylbutanal | 69.0702 | C5H9+ | Tentative |
| 2-Methylfuran | 83.0498 | C5H7O+ | Tentative |
| 5-Methyl-2(5H)-furanone | 99.0446 | C5H7O2+ | Tentative |
| 2-Methyl-6-propyl-pyrazine | 137.0983 | C8H13N2+ | Tentative |
| Acetaldehyde | 45.0325 | C2H5O+ | Tentative |
| Ethanol | 48.0323 | C2H7O+ | Tentative |
| 2,5-Diethylpyrazine | 137.0983 | C8H13N2+ | Tentative |
| 2,6-Diethylpyrazine | |||
| 3-Ethyl-2,5-dimethylpyrazine | |||
| 2-Ethyl-3,5-dimethylpyrazine | |||
| 5-Ethyl-2,3-dimethylpyrazine | |||
| 3-Ethyl-2,6-dimethylpyrazine | |||
| 2-Ethyl-3,6-dimethylpyrazine |
| Origin Based on the PTR-ToF-MS Pattern of Release of Volatile Compounds to the Oven during Baking | Possible Volatile Compounds |
|---|---|
| Fermentation origin | 3-methyl-1-butanol, 2-methyl-1-butanol, 1-pentanol, 2,3-butanedione, 3-methylbutanal, 2-methylbutanal, acetaldehyde, ethanol |
| Lipids oxidation origin | 1-hexanol, hexanal, heptanal, 1-octen-3-ol, nonanal, 2,4-decadienal, 3-penten-2-ol |
| Esters origin | ethyl octanoate, ethyl hexanoate and hexyl acetate |
| Acids origin | hexanoic acid |
| Fermentation-Maillard high retention in bread origin | phenylethyl alcohol, phenylacetaldehyde, benzaldehyde, benzyl alcohol, 3-methyl-butanoic acid and 2-methyl-butanoic acid |
| Fermentation-Maillard low retention in bread origin | acetic acid, butanoic acid and acetoin |
| Early Maillard origin | 1-methyl-pyrrol, limonene, 2,3-diethyl-5-methylpyrazine, 2-ethyl-3-methylpyrazine, 2-acetylpyrazine, 2,3-diethylpyrazine, 2,5-diethylpyrazine, 2,6-diethylpyrazine, 3-ethyl-2,5-dimethylpyrazine, 2-ethyl-3,5-dimethylpyrazine, 5-ethyl-2,3-dimethylpyrazine, 3-ethyl-2,6-dimethylpyrazine and 2-ethyl-3,6-dimethylpyrazine |
| Late Maillard origin | 2-acetyl-1-pyrroline, 2-ethylpyrazine, 2,3-dimethylpyrazine, 2,5-dimethylpyrazine and 2,6-dimethylpyrazine |
| Caramelization-Maillard origin | furan, furfuryl alcohol, furfural, 5-methyl-2(5H)-furanone, 5-methyl-furfural, 2-methylfuran |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pico, J.; Khomenko, I.; Capozzi, V.; Navarini, L.; Biasioli, F. Real-Time Monitoring of Volatile Compounds Losses in the Oven during Baking and Toasting of Gluten-Free Bread Doughs: A PTR-MS Evidence. Foods 2020, 9, 1498. https://doi.org/10.3390/foods9101498
Pico J, Khomenko I, Capozzi V, Navarini L, Biasioli F. Real-Time Monitoring of Volatile Compounds Losses in the Oven during Baking and Toasting of Gluten-Free Bread Doughs: A PTR-MS Evidence. Foods. 2020; 9(10):1498. https://doi.org/10.3390/foods9101498
Chicago/Turabian StylePico, Joana, Iuliia Khomenko, Vittorio Capozzi, Luciano Navarini, and Franco Biasioli. 2020. "Real-Time Monitoring of Volatile Compounds Losses in the Oven during Baking and Toasting of Gluten-Free Bread Doughs: A PTR-MS Evidence" Foods 9, no. 10: 1498. https://doi.org/10.3390/foods9101498