Analysis of Volatile Compounds in Soju, a Korean Distilled Spirit, by SPME-Arrow-GC/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
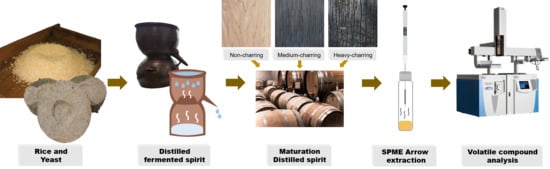
2.2. Manufacture of Distilled Spirits
2.3. Manufacture of Korean Oak Casks and Maturation of Distilled Spirits
2.4. Headspace Solid-Phase Microextraction Arrow Procedure
2.5. Gas Chromatography-Mass Spectrometry Analysis
2.6. Statistical Analysis
3. Results
3.1. Optimization of SPME Arrow Fibers
3.2. Volatile Compounds of Soju
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, G.; James, K.J.; MacNamara, K.; Stack, M.A. Characterisation of whiskeys using solid-phase microextraction with gas chromatography–mass spectrometry. J. Chromatogr. A 2000, 896, 351–359. [Google Scholar] [CrossRef]
- Hong, Y.; Park, S.K.; Choi, E.H. Flavor characteristics of Korean traditional distilled liquors produced by the co-culture of Saccharomyces and Hansenula. Microbiol. Biotechnol. Lett. 1999, 27, 236–245. [Google Scholar]
- Yi, H.C.; Moon, S.-H.; Park, J.S.; Jung, J.W.; Hwang, K.T. Volatile compounds in liquor distilled from mash produced using koji or nuruk under reduced or atmospheric pressure. J. Korean Soc. Food Sci. Nutr. 2010, 39, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Heras, M.; González-Huerta, C.; Herrera, P.; González-Sanjosé, M.L. Changes in wine volatile compounds of varietal wines during ageing in wood barrels. Anal. Chim. Acta 2004, 513, 341–350. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P.-L. Relation between volatile composition, ellagitannin content and sensory perception of oak wood chips representing different toasting processes. Eur. Food Res. Technol. 2013, 236, 735–746. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; Jalocha, J. Volatile compounds in a Spanish red wine aged in barrels made of Spanish, French, and American oak wood. J. Agric. Food Chem. 2003, 51, 7671–7678. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; del Álamo, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef]
- Ancín, C.; Garde, T.; Torrea, D.; Jimenez, N. Extraction of volatile compounds in model wine from different oak woods: Effect of SO2. Food Res. Int. 2004, 37, 375–383. [Google Scholar] [CrossRef]
- Byun, J.G.; Lee, W.K.; Nor, D.K.; Kim, S.H.; Choi, K.J.; Lee, Y.J. The relationship between tree radial growth and topographic and climatic factors in red pine and oak in central regions of Korea. J. Korean For. Soc. 2010, 99, 908–913. [Google Scholar]
- Blanch, G.P.; Tabera, J.; Herraiz, M.; Reglero, G. Preconcentration of volatile components of foods: Optimization of the steam distillation-solvent extraction at normal pressure. J. Chromatogr. A 1993, 628, 261–268. [Google Scholar] [CrossRef]
- Chen, F.; Zu, Y.; Yang, L. A novel approach for isolation of essential oil from fresh leaves of Magnolia sieboldii using microwave-assisted simultaneous distillation and extraction. Sep. Purif. Technol. 2015, 154, 271–280. [Google Scholar] [CrossRef]
- Cadwallader, K.R.; Surakarnkul, R.; Yang, S.P.; Webb, T.E. Character-impact aroma components of coriander (Coriandrum sativum L.) herb. In Flavor Chemistry of Ethnic Foods; Shahidi, F., Ho, C.T., Eds.; Springer: Boston, MA, USA, 1999; pp. 77–84. [Google Scholar]
- Wanakhachornkrai, P.; Lertsiri, S. Comparison of determination method for volatile compounds in Thai soy sauce. Food Chem. 2003, 83, 619–629. [Google Scholar] [CrossRef]
- Jo, Y.J.; Cho, I.H.; Song, C.K.; Shin, H.W.; Kim, Y.S. Comparison of fermented soybean paste (Doenjang) prepared by different methods based on profiling of volatile compounds. J. Food Sci. 2011, 76, C368–C379. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Moon, J.K.; Shibamoto, T. Analysis and antioxidant activity of extracts from broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2015, 63, 1169–1174. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, H.W.; Lee, K.G. Sensory and instrumental volatile flavor analysis of commercial orange juices prepared by different processing methods. Food Chem. 2018, 267, 217–222. [Google Scholar] [CrossRef]
- Lee, J.; Jang, H.W.; Jeong, M.C.; Yoo, S.; Ha, J. Analysis of volatile compounds as quality indicators for Fuji apples after cold storage. J. Food Biochem. 2017, 41, e12410. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, Y.Y.; Lee, K.-G.; Jang, H.W. Instrumental volatile flavor analysis of omija (Schisandra chinesis Baillon) using headspace stir-bar sorptive extraction-gas chromatography-mass spectrometry and its relationship to human sensory perceptions. Food Res. Int. 2019, 120, 650–655. [Google Scholar] [CrossRef]
- Lee, J.; Shibamoto, T.; Ha, J.; Jang, H.W. Identification of volatile markers for the detection of adulterants in red ginseng (Panax ginseng) juice using headspace stir-bar sorptive extraction coupled with gas chromatography and mass spectrometry. J. Sep. Sci. 2018, 41, 2903–2912. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.-M. Comparison of static headspace, headspace solid phase microextraction, headspace sorptive extraction, and direct thermal desorption techniques on chemical composition of French olive oils. J. Agric. Food Chem. 2003, 51, 7709–7716. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, M. Developments in multiple headspace extraction. J. Biochem. Biophys. Methods 2007, 70, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Cordero, C.; Liberto, E.; Sgorbini, B.; Rubiolo, P. Headspace sampling of the volatile fraction of vegetable matrices. J. Chromatogr. A 2008, 1184, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, W.S.; Lee, Y.Y.; Choi, Y.S.; Choi, H.; Jang, H.W. Solid-phase microextraction Arrow for the volatile organic compounds in soy sauce. J. Sep. Sci. 2019, 42, 2942–2948. [Google Scholar] [CrossRef]
- Song, N.E.; Lee, J.Y.; Lee, Y.Y.; Park, J.D.; Jang, H.W. Comparison of headspace–SPME and SPME-Arrow–GC–MS methods for the determination of volatile compounds in Korean salt–fermented fish sauce. Appl. Biol. Chem. 2019, 62, 16. [Google Scholar] [CrossRef] [Green Version]
- Nam, T.G.; Lee, J.Y.; Kim, B.K.; Song, N.E.; Jang, H.W. Analyzing volatiles in brown rice vinegar by headspace solid-phase microextraction (SPME)–Arrow: Optimizing the extraction conditions and comparisons with conventional SPME. Int. J. Food Prop. 2019, 22, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. PAL SPME Arrow—Evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal. Bioanal. Chem. 2016, 408, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Helin, A.; Rönkkö, T.; Parshintsev, J.; Hartonen, K.; Schilling, B.; Läubli, T.; Riekkola, M.-L. Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J. Chromatogr. A 2015, 1426, 56–63. [Google Scholar] [CrossRef]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting molecules in complex matrices with spme arrows: A review. Separations 2020, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Castro, Ó.; Trabalón, L.; Schilling, B.; Borrull, F.; Pocurull, E. Solid phase microextraction Arrow for the determination of synthetic musk fragrances in fish samples. J. Chromatogr. A 2019, 1591, 55–61. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.Y.; Choi, Y.S.; Sung, J.M.; Jang, H.W. Comparison of different types of SPME Arrow sorbents to analyze volatile compounds in Cirsium setidens Nakai. Foods 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Germain-Robin, H. The Maturation of Distilled Spirits: Vision & Patience; White Mule Press: Hayward, CA, USA, 2016; pp. 13–58. [Google Scholar]
- Buxton, I.; Hughes, P.S. The Science and Commerce of Whisky; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 134–168. [Google Scholar]
- Câmara, J.S.; Marques, J.C.; Perestrelo, R.M.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M. Comparative study of the whisky aroma profile based on headspace solid phase microextraction using different fibre coatings. J. Chromatogr. A 2007, 1150, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, M. Properties of Commercial SPME Coatings. Applications of Solid Phase Microextraction; Pawliszyn, J., Ed.; Royal Society of Chemistry: Hertfordshire, UK, 1999; ISBN 0-85404-525-2. [Google Scholar]
- Bartowsky, E.J.; Henschke, P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.M.d.V.; Vilela, L.D.F.; Santos, C.; Lima, N.; Schwan, R.F. Volatile compounds and protein profiles analyses of fermented cocoa beans and chocolates from different hybrids cultivated in Brazil. Food Res. Int. 2018, 109, 196–203. [Google Scholar] [CrossRef]
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C.; Lepoutre, J.P.; Gunata, Z. Volatile composition of red wine from cv. Kalecik Karasι grown in central Anatolia. Food Chem. 2004, 85, 207–213. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Rodríguez Mozaz, S.; Ancín Azpilicueta, C. Volatile composition of aged wine in used barrels of French oak and of American oak. Food Res. Int. 2002, 35, 603–610. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Sauvageot, F.; Feuillat, F. The influence of oak wood (Quercus robur L., Q. petraea Liebl.) on the flavor of burgundy pinot noir. An examination of variation among idividual trees. Am. J. Enol. Vitic. 1999, 50, 447–455. [Google Scholar]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-active compounds of American, French, Hungarian and Russian oak woods, studied by GC–MS and GC–O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, J.; Chin, Y.-W.; Lee, J.-Y.; Kim, T.-W.; Jang, H.W. Analysis of Volatile Compounds in Soju, a Korean Distilled Spirit, by SPME-Arrow-GC/MS. Foods 2020, 9, 1422. https://doi.org/10.3390/foods9101422
Cha J, Chin Y-W, Lee J-Y, Kim T-W, Jang HW. Analysis of Volatile Compounds in Soju, a Korean Distilled Spirit, by SPME-Arrow-GC/MS. Foods. 2020; 9(10):1422. https://doi.org/10.3390/foods9101422
Chicago/Turabian StyleCha, Jiyoon, Young-Wook Chin, Jun-Young Lee, Tae-Wan Kim, and Hae Won Jang. 2020. "Analysis of Volatile Compounds in Soju, a Korean Distilled Spirit, by SPME-Arrow-GC/MS" Foods 9, no. 10: 1422. https://doi.org/10.3390/foods9101422